Discontinuation and Switchback After Non-Medical Switching from Originator Tumor Necrosis Factor Alpha (TNF) Inhibitors to Biosimilars: A Meta-Analysis of Real-World Studies from 2012 to 2018
- PMID: 35737227
- PMCID: PMC9309144
- DOI: 10.1007/s12325-022-02173-7
Discontinuation and Switchback After Non-Medical Switching from Originator Tumor Necrosis Factor Alpha (TNF) Inhibitors to Biosimilars: A Meta-Analysis of Real-World Studies from 2012 to 2018
Erratum in
-
Correction to: Discontinuation and Switchback After Non-Medical Switching from Originator Tumor Necrosis Factor Alpha (TNF) Inhibitors to Biosimilars: A Meta-Analysis of Real-World Studies from 2012 to 2018.Adv Ther. 2022 Nov;39(11):5300. doi: 10.1007/s12325-022-02255-6. Adv Ther. 2022. PMID: 36040636 Free PMC article. No abstract available.
Abstract
Introduction: To examine the prevalence rates of biosimilar discontinuation and switchback to the originator tumor necrosis factor alpha (TNF) inhibitors following non-medical switch (NMS) in patients.
Methods: Real-world studies reporting biosimilar discontinuation and switchback rates following NMS published between January 2012 and August 2018 were identified through a systematic literature review. A meta-analysis estimated the annualized discontinuation and switchback rates. A subsequent meta-analysis assessed annualized incremental discontinuation rate among studies reporting both discontinuation rates in patients who underwent an NMS (switchers) and patients who remained on originators (non-switchers).
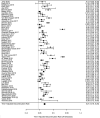
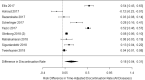
Results: A total of 66 publications were identified: 31 in gastroenterology, 32 in rheumatology, and 3 in both. Half of the studies reported switchback rates; only 9 studies reported discontinuation rates for both switchers and non-switchers. Across studies, the mean/range sample size of the NMS patient population was 136/9-1641; mean/range follow-up was 10/3-24 months. Annualized biosimilar discontinuation rate was 21% (95% confidence interval [CI] 18%, 25%). Switchback rate was 14% (95% CI 10%, 17%) among all NMS patients and 62% (95% CI 44%, 80%) among discontinuers. The mean/range sample size of switchers and non-switchers was 344/89-1621 and 768/19-2870, respectively; mean/range follow-up was 11/6-18 and 12/6-8 months, respectively. Annualized incremental biosimilar discontinuation rate was 18% (95% CI 4%, 31%).
Conclusion: Biosimilar discontinuation was found to be prevalent among patients who underwent an NMS from an originator TNF inhibitor to its biosimilar(s) in the real world. In addition, switchback to the originator TNF inhibitors was common following biosimilar discontinuation. Careful consideration is necessary when switching patients already on an originator TNF inhibitor to its biosimilar(s). Main limitations included the heterogeneity of the studies and the limited comparability of the data.
Keywords: Biosimilar; Discontinuation; Non-medical switch; Switchback; TNF inhibitors.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Figures


Similar articles
-
The Economic Impact of Originator-to-Biosimilar Non-medical Switching in the Real-World Setting: A Systematic Literature Review.Adv Ther. 2022 Jan;39(1):455-487. doi: 10.1007/s12325-021-01951-z. Epub 2021 Nov 15. Adv Ther. 2022. PMID: 34780028 Free PMC article.
-
Switching from originator infliximab to biosimilar versus continuing on originator in inflammatory bowel disease: results from the observational Project NORTH study.Scand J Gastroenterol. 2022 Dec;57(12):1435-1442. doi: 10.1080/00365521.2022.2090275. Epub 2022 Jul 14. Scand J Gastroenterol. 2022. PMID: 35833832
-
Effectiveness and Safety of Switching Originator and Biosimilar Epoetins in Patients with Chronic Kidney Disease in a Large-Scale Italian Cohort Study.Drug Saf. 2019 Dec;42(12):1437-1447. doi: 10.1007/s40264-019-00845-y. Drug Saf. 2019. PMID: 31228010 Free PMC article.
-
Uptake of tumour necrosis factor-alpha inhibitor biosimilars for psoriasis: a drug utilization study from the British Association of Dermatologists Biologic and Immunomodulators Register (BADBIR).Br J Dermatol. 2023 Jul 7;189(1):62-70. doi: 10.1093/bjd/ljad107. Br J Dermatol. 2023. PMID: 37016153
-
Non-medical Switching from Originator Tumor Necrosis Factor Inhibitors to Their Biosimilars: Systematic Review of Randomized Controlled Trials and Real-World Studies.Adv Ther. 2018 Sep;35(9):1295-1332. doi: 10.1007/s12325-018-0742-9. Epub 2018 Aug 6. Adv Ther. 2018. PMID: 30084060 Free PMC article.
Cited by
-
Health Care Providers' Experiences of a Mandatory Nationwide Transition to an Adalimumab Biosimilar.ACR Open Rheumatol. 2023 Dec;5(12):644-651. doi: 10.1002/acr2.11617. Epub 2023 Oct 1. ACR Open Rheumatol. 2023. PMID: 37779361 Free PMC article.
-
Modeling the Effects of Formulary Exclusions: How Many Patients Could Be Affected by a Specific Exclusion?J Health Econ Outcomes Res. 2024 Mar 25;11(1):86-93. doi: 10.36469/001c.94544. eCollection 2024. J Health Econ Outcomes Res. 2024. PMID: 38544720 Free PMC article.
References
-
- Biosimilars Resource Center. What are biologics? 2017. https://www.biosimilarsresourcecenter.org/faq/what-are-biologics/. Accessed 16 Oct 2020.
-
- Singh SC, Bagnato KM. The economic implications of biosimilars. Am J Manag Care. 2015;21:s331–s340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

