Irisin Attenuates Pathological Neovascularization in Oxygen-Induced Retinopathy Mice
- PMID: 35737379
- PMCID: PMC9233294
- DOI: 10.1167/iovs.63.6.21
Irisin Attenuates Pathological Neovascularization in Oxygen-Induced Retinopathy Mice
Abstract
Purpose: Abnormal angiogenesis is a defining feature in a couple of ocular neovascular diseases. The application of anti-VEGFA therapy has achieved certain benefits in the clinic, accompanying side effects and poor responsiveness in many patients. The present study investigated the role of irisin in retinal neovascularization.
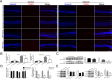
Methods: Western blot and quantitative PCR were used to determine irisin expression in the oxygen-induced retinopathy mice model. The pathological angiogenesis and inflammation index were examined after irisin administration. Primary retinal astrocytes were cultured and analyzed for VEGFA expression in vitro. Astrocyte-conditioned medium was collected for transwell assay and tube formation assay in human microvascular endothelial cells-1.
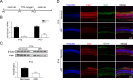
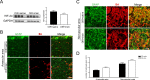
Results: Irisin was downregulated in the oxygen-induced retinopathy mice retinae. Additional irisin attenuated pathological angiogenesis, inflammation, and apoptosis in vivo. In vitro, irisin decreased astrocyte VEGFA production, and the conditioned medium suppressed human microvascular endothelial cells-1 migration. Last, irisin inhibited hypoxia-inducible factor-2α, nuclear factor-κB, and pNF-κB (Phospho-Nuclear Factor-κB) expression.
Conclusions: Irisin mitigates retinal pathological angiogenesis.
Chinese Abstract.
Conflict of interest statement
Disclosure:
Figures




Similar articles
-
Imidazole-based alkaloid derivative LCB54-0009 suppresses ocular angiogenesis and lymphangiogenesis in models of experimental retinopathy and corneal neovascularization.Br J Pharmacol. 2015 Aug;172(15):3875-89. doi: 10.1111/bph.13177. Epub 2015 Jun 26. Br J Pharmacol. 2015. PMID: 25917462 Free PMC article.
-
Antiangiogenic effect of betaine on pathologic retinal neovascularization via suppression of reactive oxygen species mediated vascular endothelial growth factor signaling.Vascul Pharmacol. 2017 Mar;90:19-26. doi: 10.1016/j.vph.2016.07.007. Epub 2016 Jul 27. Vascul Pharmacol. 2017. PMID: 27473515
-
Endothelial deletion of phospholipase D2 reduces hypoxic response and pathological angiogenesis.Arterioscler Thromb Vasc Biol. 2014 Aug;34(8):1697-703. doi: 10.1161/ATVBAHA.114.303416. Epub 2014 Jun 19. Arterioscler Thromb Vasc Biol. 2014. PMID: 24947526
-
CircPDE4B inhibits retinal pathological angiogenesis via promoting degradation of HIF-1α though targeting miR-181c.IUBMB Life. 2020 Sep;72(9):1920-1929. doi: 10.1002/iub.2307. Epub 2020 Jun 25. IUBMB Life. 2020. PMID: 32584521
-
Apatinib, an Inhibitor of Vascular Endothelial Growth Factor Receptor 2, Suppresses Pathologic Ocular Neovascularization in Mice.Invest Ophthalmol Vis Sci. 2017 Jul 1;58(9):3592-3599. doi: 10.1167/iovs.17-21416. Invest Ophthalmol Vis Sci. 2017. PMID: 28715845
Cited by
-
On implications of somatostatin in diabetic retinopathy.Neural Regen Res. 2024 Sep 1;19(9):1984-1990. doi: 10.4103/1673-5374.390955. Epub 2023 Dec 15. Neural Regen Res. 2024. PMID: 38227526 Free PMC article.
-
Effect of astrocyte GPER on the optic nerve inflammatory response following optic nerve injury in mice.Heliyon. 2024 Apr 10;10(8):e29428. doi: 10.1016/j.heliyon.2024.e29428. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38638966 Free PMC article.
-
Effects and potential mechanisms of exercise and physical activity on eye health and ocular diseases.Front Med (Lausanne). 2024 Mar 22;11:1353624. doi: 10.3389/fmed.2024.1353624. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38585147 Free PMC article. Review.
-
Single-Cell Multiomics Profiling Reveals Heterogeneity of Müller Cells in the Oxygen-Induced Retinopathy Model.Invest Ophthalmol Vis Sci. 2024 Nov 4;65(13):8. doi: 10.1167/iovs.65.13.8. Invest Ophthalmol Vis Sci. 2024. PMID: 39504047 Free PMC article.
References
-
- Mca B, Sldbc D.. Current perspectives on established and novel therapies for pathological neovascularization in retinal disease. Biochem Pharmacol . 2019; 164: 321–325. - PubMed
-
- Qiu B, Tan A, Veluchamy AB, et al. .. Apratoxin S4 inspired by a marine natural product, a new treatment option for ocular angiogenic diseases. Invest Ophthalmol Vis Sci. 2019; 60(8): 3254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

