Adaptive Dynamic Therapy and Survivorship for Operable Pancreatic Cancer
- PMID: 35737385
- PMCID: PMC9227002
- DOI: 10.1001/jamanetworkopen.2022.18355
Adaptive Dynamic Therapy and Survivorship for Operable Pancreatic Cancer
Erratum in
-
Errors in Figure 2.JAMA Netw Open. 2022 Jul 1;5(7):e2227318. doi: 10.1001/jamanetworkopen.2022.27318. JAMA Netw Open. 2022. PMID: 35877129 Free PMC article. No abstract available.
Abstract
Importance: Neoadjuvant therapy is increasingly used in localized pancreatic carcinoma, and survival is correlated with carbohydrate antigen 19-9 (CA19-9) levels and histopathologic response following neoadjuvant therapy. With several regimens now available, the choice of chemotherapy could be best dictated by response to neoadjuvant therapy (as measured by CA19-9 levels and/or pathologic response), a strategy defined herein as adaptive dynamic therapy.
Objective: To evaluate the association of adaptive dynamic therapy with oncologic outcomes in patients with surgically resected pancreatic cancer.
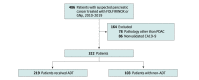
Design, setting, and participants: This retrospective cohort study included patients with localized pancreatic cancer who were treated with either gemcitabine/nab-paclitaxel or fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) preoperatively between 2010 and 2019 at a high-volume tertiary care academic center. Participants were identified from a prospectively maintained database and had a median follow-up of 49 months. Data were analyzed from October 17 to November 24, 2020.
Exposures: The adaptive dynamic therapy group included 219 patients who remained on or switched to an alternative regimen as dictated by CA19-9 response and for whom the adjuvant regimen was selected based on CA19-9 and/or pathologic response. The nonadaptive dynamic therapy group included 103 patients who had their chemotherapeutic regimen selected independent of CA19-9 and/or tumoral response.
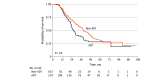
Main outcomes and measures: Prognostic implications of dynamic perioperative therapy assessed through Kaplan-Meier analysis, Cox regression, and inverse probability weighted estimators.
Results: A total of 322 consecutive patients (mean [SD] age, 65.1 [9] years; 162 [50%] women) were identified. The adaptive dynamic therapy group, compared with the nonadaptive dynamic therapy group, had a more pronounced median (IQR) decrease in CA19-9 levels (-80% [-92% to -56%] vs -45% [-81% to -13%]; P < .001), higher incidence of complete or near-complete tumoral response (25 [12%] vs 2 [2%]; P = .007), and lower median (IQR) number of lymph node metastasis (1 [0 to 4] vs 2 [0 to 4]; P = .046). Overall survival was significantly improved in the dynamic group compared with the nondynamic group (38.7 months [95% CI, 34.0 to 46.7 months] vs 26.5 months [95% CI, 23.5 to 32.9 months]; P = .03), and on adjusted analysis, dynamic therapy was independently associated with improved survival (hazard ratio, 0.73; 95% CI, 0.53 to 0.99; P = .04). On inverse probability weighted analysis of 320 matched patients, the average treatment effect of dynamic therapy was to increase overall survival by 11.1 months (95% CI, 1.5 to 20.7 months; P = .02).
Conclusions and relevance: In this cohort study that sought to evaluate the role of adaptive dynamic therapy in localized pancreatic cancer, selecting a chemotherapeutic regimen based on response to preoperative therapy was associated with improved survival. These findings support an individualized and in vivo assessment of response to perioperative therapy in pancreatic cancer.
Conflict of interest statement
Figures
Similar articles
-
Response and Survival Associated With First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma.JAMA Surg. 2020 Sep 1;155(9):832-839. doi: 10.1001/jamasurg.2020.2286. JAMA Surg. 2020. PMID: 32667641 Free PMC article.
-
Evaluation of Adjuvant Chemotherapy Survival Outcomes Among Patients With Surgically Resected Pancreatic Carcinoma With Node-Negative Disease After Neoadjuvant Therapy.JAMA Surg. 2023 Jan 1;158(1):55-62. doi: 10.1001/jamasurg.2022.5696. JAMA Surg. 2023. PMID: 36416848 Free PMC article.
-
Evaluation of Adjuvant Chemotherapy in Patients With Resected Pancreatic Cancer After Neoadjuvant FOLFIRINOX Treatment.JAMA Oncol. 2020 Nov 1;6(11):1733-1740. doi: 10.1001/jamaoncol.2020.3537. JAMA Oncol. 2020. PMID: 32910170 Free PMC article.
-
Comparison of FOLFIRINOX vs Gemcitabine Plus Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Ductal Adenocarcinoma.JAMA Netw Open. 2022 Jun 1;5(6):e2216199. doi: 10.1001/jamanetworkopen.2022.16199. JAMA Netw Open. 2022. PMID: 35675073 Free PMC article.
-
Meta-analysis examining overall survival in patients with pancreatic cancer treated with second-line 5-fluorouracil and oxaliplatin-based therapy after failing first-line gemcitabine-containing therapy: effect of performance status and comparison with other regimens.BMC Cancer. 2020 Jul 8;20(1):633. doi: 10.1186/s12885-020-07110-x. BMC Cancer. 2020. PMID: 32641104 Free PMC article.
Cited by
-
CD3-CD8 immune score associated with a clinical score stratifies PDAC prognosis regardless of adjuvant or neoadjuvant chemotherapy.Oncoimmunology. 2023 Dec 21;13(1):2294563. doi: 10.1080/2162402X.2023.2294563. eCollection 2024. Oncoimmunology. 2023. PMID: 38169969 Free PMC article.
-
Pancreatic Cancer Multidisciplinary Clinic is Associated with Improved Treatment and Elimination of Socioeconomic Disparities.Ann Surg Oncol. 2024 Mar;31(3):1906-1915. doi: 10.1245/s10434-023-14609-7. Epub 2023 Nov 21. Ann Surg Oncol. 2024. PMID: 37989957
-
Adjuvant Chemotherapy After Resection of Localized Pancreatic Adenocarcinoma Following Preoperative FOLFIRINOX.JAMA Oncol. 2025 Mar 1;11(3):276-287. doi: 10.1001/jamaoncol.2024.5917. JAMA Oncol. 2025. PMID: 39847363
-
Current Treatment of Potentially Resectable Pancreatic Ductal Adenocarcinoma: A Medical Oncologist's Perspective.Cancer Control. 2023 Jan-Dec;30:10732748231173212. doi: 10.1177/10732748231173212. Cancer Control. 2023. PMID: 37115533 Free PMC article. Review.
-
Therapeutic potential of mangiferin in cancer: Unveiling regulatory pathways, mechanisms of action, and bioavailability enhancements - An updated review.Food Sci Nutr. 2023 Dec 20;12(3):1413-1429. doi: 10.1002/fsn3.3869. eCollection 2024 Mar. Food Sci Nutr. 2023. PMID: 38455223 Free PMC article. Review.
References
-
- Sinn M, Bahra M, Liersch T, et al. . CONKO-005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: a multicenter randomized phase III trial. J Clin Oncol. 2017;35(29):3330-3337. doi:10.1200/JCO.2017.72.6463 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

