Repeatability and Discriminatory Power of Chart-Based Visual Function Tests in Individuals With Age-Related Macular Degeneration: A MACUSTAR Study Report
- PMID: 35737401
- PMCID: PMC9227684
- DOI: 10.1001/jamaophthalmol.2022.2113
Repeatability and Discriminatory Power of Chart-Based Visual Function Tests in Individuals With Age-Related Macular Degeneration: A MACUSTAR Study Report
Abstract
Importance: There is a need for validated clinical end points that are reliably able to quantify potential therapeutic effects of future treatments targeting age-related macular degeneration (AMD) before the onset of serious visual impairment.
Objective: To assess the reliability and discriminatory power of 5 simple chart-based visual function (VF) tests as potential measures for clinical trial end points with regulatory and patient-access intention in intermediate AMD (iAMD).
Design, setting, and participants: This international noninterventional study took place at 18 tertiary ophthalmology departments across Europe. Participants were recruited between April 2018 and March 2020 and were identified during routine clinical review. Participants with no AMD and early AMD were recruited from hospital staff, friends, and family of participants with AMD and via referrals from community ophthalmologists and optometrists. The repeatability and discriminatory power of 5 simple chart-based assessments of VF (best-corrected visual acuity [BCVA], low-luminance visual acuity [LLVA], Moorfields Acuity Test [MAT], Pelli-Robson Contrast Sensitivity [CS], and International Reading Speed Test [IReST]) were assessed in a repeated-measures design. VF assessments were performed on day 0 and day 14. Participants with early AMD, iAMD, late AMD, and no AMD were recruited.
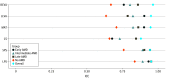
Main outcomes and measures: Intraclass correlation coefficients (ICCs) and Bland-Altman 95% limits of agreement (LoA) were computed to assess repeatability. Area under the receiver operating characteristic curves (AUCs) determined the discriminatory ability of all measures to classify individuals as having no AMD or iAMD and to differentiate iAMD from its neighboring disease states.
Results: A total of 301 participants (mean [SD] age, 71 [7] years; 187 female participants [62.1%]) were included in the study. Thirty-four participants (11.3%) had early AMD, 168 (55.8%) had iAMD, 43 (14.3%) had late AMD, and 56 (18.6%) had no AMD. ICCs for all VF measures ranged between 0.88 and 0.96 when all participants were considered, indicating good to excellent repeatability. All measures displayed excellent discrimination between iAMD and late AMD (AUC, 0.92-0.99). Early AMD was indistinguishable from iAMD on all measures (AUC, 0.54-0.64). CS afforded the best discrimination between no AMD and iAMD (AUC, 0.77). Under the same conditions, BCVA, LLVA, and MAT were fair discriminators (AUC, 0.69-0.71), and IReST had poor discrimination (AUC, 0.57-0.61).
Conclusions and relevance: BCVA, LLVA, MAT, CS, and IReST had adequate repeatability in this multicenter, multiexaminer setting but limited power to discriminate between no AMD and iAMD. The prognostic power of these variables to predict conversion from iAMD to late AMD is being examined in the ongoing longitudinal part of the MACUSTAR study.
Conflict of interest statement
Figures

Comment in
-
MACUSTAR-Do We Have Accurate and Precise Visual Function Outcome Measures Needed to Conduct a Multicenter Clinical Trial of an Early Treatment for Non-Neovascular Age-Related Macular Degeneration?JAMA Ophthalmol. 2022 Aug 1;140(8):789-790. doi: 10.1001/jamaophthalmol.2022.2134. JAMA Ophthalmol. 2022. PMID: 35737400 No abstract available.
References
-
- Lotery A, Xu X, Zlatava G, Loftus J. Burden of illness, visual impairment and health resource utilisation of patients with neovascular age-related macular degeneration: results from the UK cohort of a five-country cross-sectional study. Br J Ophthalmol. 2007;91(10):1303-1307. doi: 10.1136/bjo.2007.116939 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

