Ojeok-san ameliorates visceral and somatic nociception in a mouse model of colitis induced colorectal cancer
- PMID: 35737651
- PMCID: PMC9223640
- DOI: 10.1371/journal.pone.0270338
Ojeok-san ameliorates visceral and somatic nociception in a mouse model of colitis induced colorectal cancer
Abstract
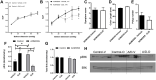
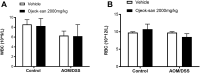
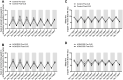
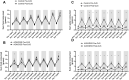
Cancer patients can develop visceral, somatic, and neuropathic pain, largely due to the malignancy itself and its treatments. Often cancer patients and survivors turn to the use of complementary and alternative medicine (CAM) to alleviate pain and fatigue. Thus, it is necessary to investigate how CAM therapies work as novel analgesics to treat cancer pain. Ojeok-san (OJS) is an herbal formula consisting of seventeen herbs. This herbal formula has been shown to possess anti-inflammatory, immunoregulatory, and analgesic properties. In this study, we examined the potential beneficial effects and mechanism of action of OJS in a preclinical model of colitis-associated colorectal cancer. Male and female C57BL/6J mice were exposed to the carcinogen, azoxymethane (AOM, 10 mg/kg) and a chemical inflammatory driver, dextran sulfate sodium (DSS1-2%), to promote tumorigenesis in the colorectum. OJS was given orally (500, 1000, and 2000 mg/kg) to determine its influence on disease activity, tumor burden, nociception, sedation, Erk signaling, and behavioral and metabolic outcomes. In addition, in vitro studies were performed to assess CT-26 cell viability, dorsal root ganglia (DRG) activation, and bone-marrow-derived macrophage (BMDM) inflammatory response to lipopolysaccharide stimulation after OJS treatment. We found that administration of 2000 mg/kg of OJS was able to mitigate mechanical somatic and visceral nociception via Erk signaling without affecting symptom score and polyp number. Moreover, we discovered that OJS has sedative properties and elicits prolonged total sleeping time in AOM/DSS mice. Our in vitro experiments showed that OJS has the capacity to reduce TNFα gene expression in LPS-stimulated BMDM, but no changes were observed in DRG spike number and CT-26 cell proliferation. Taken together, these data suggest that OJS ameliorates nociception in mice and warrants further examination as a potential CAM therapy to promote analgesia.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Society AC. American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. Atlanta; 2017.
-
- Corporation NHI. National Health Insurance Corporation Health Insurance Review Assessment Service: Samwon Press, Seoul Korea; 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

