Punctual and kinetic MRD analysis from the Fondazione Italiana Linfomi MCL0208 phase 3 trial in mantle cell lymphoma
- PMID: 35737911
- PMCID: PMC9507010
- DOI: 10.1182/blood.2021014270
Punctual and kinetic MRD analysis from the Fondazione Italiana Linfomi MCL0208 phase 3 trial in mantle cell lymphoma
Abstract
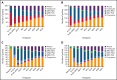
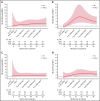
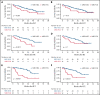
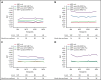
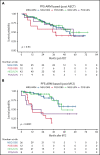
Minimal residual disease (MRD) analysis is a known predictive tool in mantle cell lymphoma (MCL). We describe MRD results from the Fondazione Italiana Linfomi phase 3 MCL0208 prospective clinical trial assessing lenalidomide (LEN) maintenance vs observation after autologous stem cell transplantation (ASCT) in the first prospective comprehensive analysis of different techniques, molecular markers, and tissues (peripheral blood [PB] and bone marrow [BM]), taken at well-defined time points. Among the 300 patients enrolled, a molecular marker was identified in 250 (83%), allowing us to analyze 234 patients and 4351 analytical findings from 10 time points. ASCT induced high rates of molecular remission (91% in PB and 83% in BM, by quantitative real-time polymerase chain reaction [RQ-PCR]). Nevertheless, the number of patients with persistent clinical and molecular remission decreased over time in both arms (up to 30% after 36 months). MRD predicted early progression and long-term outcome, particularly from 6 months after ASCT (6-month time to progression [TTP] hazard ratio [HR], 3.83; P < .001). In single-timepoint analysis, BM outperformed PB, and RQ-PCR was more reliable, while nested PCR appeared applicable to a larger number of patients (234 vs 176). To improve MRD performance, we developed a time-varying kinetic model based on regularly updated MRD results and the MIPI (Mantle Cell Lymphoma International Prognostic Index), showing an area under the ROC (Receiver Operating Characteristic) curve (AUROC) of up to 0.87 using BM. Most notably, PB reached an AUROC of up to 0.81; with kinetic analysis, it was comparable to BM in performance. MRD is a powerful predictor over the entire natural history of MCL and is suitable for models with a continuous adaptation of patient risk. The study can be found in EudraCT N. 2009-012807-25 (https://eudract.ema.europa.eu/).
© 2022 by The American Society of Hematology.
Figures

Comment in
-
Predicting the future in MCL with MRD.Blood. 2022 Sep 22;140(12):1332-1333. doi: 10.1182/blood.2022017278. Blood. 2022. PMID: 36136360 No abstract available.
References
-
- Herrmann A, Hoster E, Zwingers T, et al. . Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27(4):511-518. - PubMed
-
- Hermine O, Hoster E, Walewski J, et al. ; European Mantle Cell Lymphoma Network . Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388(10044):565-575. - PubMed
-
- Le Gouill S, Thieblemont C, Oberic L, et al. ; LYSA Group . Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377(13): 1250-1260. - PubMed
-
- Ladetto M, Cortelazzo S, Ferrero S, et al. . Lenalidomide maintenance after autologous haematopoietic stem-cell transplantation in mantle cell lymphoma: results of a Fondazione Italiana Linfomi (FIL) multicentre, randomised, phase 3 trial. Lancet Haematol. 2021;8(1):e34-e44. - PubMed
-
- Ferrero S, Grimaldi D, Dreyling M. Tailored treatment in mantle cell lymphoma. Ann Lymphoma. 2020;4:12.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

