Liver-to-lung microembolic NETs promote gasdermin D-dependent inflammatory lung injury in sickle cell disease
- PMID: 35737916
- PMCID: PMC9437711
- DOI: 10.1182/blood.2021014552
Liver-to-lung microembolic NETs promote gasdermin D-dependent inflammatory lung injury in sickle cell disease
Abstract
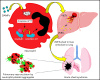
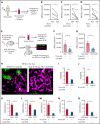
Acute lung injury, referred to as the acute chest syndrome, is a major cause of morbidity and mortality in patients with sickle cell disease (SCD), which often occurs in the setting of a vaso-occlusive painful crisis. P-selectin antibody therapy reduces hospitalization of patients with SCD by ∼50%, suggesting that an unknown P-selectin-independent mechanism promotes remaining vaso-occlusive events. In patients with SCD, intraerythrocytic polymerization of mutant hemoglobin promotes ischemia-reperfusion injury and hemolysis, which leads to the development of sterile inflammation. Using intravital microscopy in transgenic, humanized mice with SCD and in vitro studies with blood from patients with SCD, we reveal for the first time that the sterile inflammatory milieu in SCD promotes caspase-4/11-dependent activation of neutrophil-gasdermin D (GSDMD), which triggers P-selectin-independent shedding of neutrophil extracellular traps (NETs) in the liver. Remarkably, these NETs travel intravascularly from liver to lung, where they promote neutrophil-platelet aggregation and the development of acute lung injury. This study introduces a novel paradigm that liver-to-lung embolic translocation of NETs promotes pulmonary vascular vaso-occlusion and identifies a new GSDMD-mediated, P-selectin-independent mechanism of lung injury in SCD.
© 2022 by The American Society of Hematology.
Figures







Comment in
-
NETs in sickle cell disease, quo vadis?Blood. 2022 Sep 1;140(9):938-939. doi: 10.1182/blood.2022017346. Blood. 2022. PMID: 36048478 No abstract available.
References
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018-2031. - PubMed
-
- Abubakar II, Tillmann T, Banerjee A; GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117-171. - PMC - PubMed
-
- Kato GJ, Piel FB, Reid CD, et al. . Sickle cell disease. Nat Rev Dis Primers. 2018;4(1):18010. - PubMed
-
- Sundd P, Ofori-Acquah SF. Sterile inflammation in sickle cell disease. In: Gladwin MT, Novelli EM, Kato GJ, eds. Sickle Cell Disease. New York, NY: McGraw-Hill; 2021:93-108.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

