Biochemical Failure Is Not a Surrogate End Point for Overall Survival in Recurrent Prostate Cancer: Analysis of NRG Oncology/RTOG 9601
- PMID: 35737923
- PMCID: PMC9514834
- DOI: 10.1200/JCO.21.02741
Biochemical Failure Is Not a Surrogate End Point for Overall Survival in Recurrent Prostate Cancer: Analysis of NRG Oncology/RTOG 9601
Abstract
Purpose: Metastasis-free survival (MFS), but not event-free survival, is a validated surrogate end point for overall survival (OS) in men treated for localized prostate cancer. It remains unknown if this holds true in biochemically recurrent disease after radical prostatectomy. Leveraging NRG/RTOG 9601, we aimed to determine the performance of intermediate clinical end points (ICEs) as surrogate end points for OS in recurrent prostate cancer.
Materials and methods: NRG/RTOG 9601 randomly assigned 760 men with recurrence after prostatectomy to salvage radiation therapy with 2 years of placebo versus bicalutamide 150 mg daily. ICEs assessed were biochemical failure (BF) per NRG/RTOG 9601 (prostate-specific antigen nadir + 0.3-0.5 ng/mL or initiation of salvage hormone therapy; [BF1]) and NRG/RTOG 0534 (prostate-specific antigen nadir+2 ng/mL; [BF2]), distant metastasis (DM), and MFS (DM or death). Surrogacy was assessed by the Prentice criteria and a two-stage meta-analytic approach (condition one assessed at the patient level with Kendall's τ and condition two assessed by randomly dividing the entire trial cohort into 10 pseudo trial centers and calculating the average R2 between treatment hazard ratios for ICE and OS).
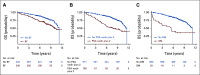
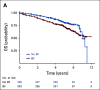
Results: BF1, BF2, DM, and MFS satisfied the four Prentice criteria. However, with the two-condition meta-analytic approach, there was strong correlation between MFS and OS (τ = 0.86), moderate correlation between DM and OS (τ = 0.66), and weaker correlation between BF1 (τ = 0.25) or BF2 (τ = 0.40) and OS. Similarly, for condition two, the treatment effect of antiandrogen therapy on MFS and OS were correlated (R2 = 0.67), but this was not true for BF1 (R2 = 0.09), BF2 (R2 = 0.12), or DM (R2 = 0.18) and OS.
Conclusion: MFS is also a strong surrogate for OS in men receiving salvage radiation therapy for recurrence after prostatectomy. Caution should be used when inferring survival benefit from effects on BF in biochemically recurrent prostate cancer. Lack of comorbidity data did not allow us to assess whether BF in men with no/minimal comorbidity could serve as a surrogate for OS.
Trial registration: ClinicalTrials.gov NCT00002874.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures


Comment in
-
Socioeconomic Factors, Urological Epidemiology and Practice Patterns.J Urol. 2023 Mar;209(3):623-625. doi: 10.1097/JU.0000000000003101. Epub 2022 Dec 15. J Urol. 2023. PMID: 36519370 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

