Transcranial magnetic stimulation of the brain: What is stimulated? - A consensus and critical position paper
- PMID: 35738037
- PMCID: PMC9753778
- DOI: 10.1016/j.clinph.2022.04.022
Transcranial magnetic stimulation of the brain: What is stimulated? - A consensus and critical position paper
Abstract
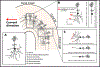
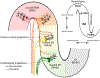
Transcranial (electro)magnetic stimulation (TMS) is currently the method of choice to non-invasively induce neural activity in the human brain. A single transcranial stimulus induces a time-varying electric field in the brain that may evoke action potentials in cortical neurons. The spatial relationship between the locally induced electric field and the stimulated neurons determines axonal depolarization. The induced electric field is influenced by the conductive properties of the tissue compartments and is strongest in the superficial parts of the targeted cortical gyri and underlying white matter. TMS likely targets axons of both excitatory and inhibitory neurons. The propensity of individual axons to fire an action potential in response to TMS depends on their geometry, myelination and spatial relation to the imposed electric field and the physiological state of the neuron. The latter is determined by its transsynaptic dendritic and somatic inputs, intrinsic membrane potential and firing rate. Modeling work suggests that the primary target of TMS is axonal terminals in the crown top and lip regions of cortical gyri. The induced electric field may additionally excite bends of myelinated axons in the juxtacortical white matter below the gyral crown. Neuronal excitation spreads ortho- and antidromically along the stimulated axons and causes secondary excitation of connected neuronal populations within local intracortical microcircuits in the target area. Axonal and transsynaptic spread of excitation also occurs along cortico-cortical and cortico-subcortical connections, impacting on neuronal activity in the targeted network. Both local and remote neural excitation depend critically on the functional state of the stimulated target area and network. TMS also causes substantial direct co-stimulation of the peripheral nervous system. Peripheral co-excitation propagates centrally in auditory and somatosensory networks, but also produces brain responses in other networks subserving multisensory integration, orienting or arousal. The complexity of the response to TMS warrants cautious interpretation of its physiological and behavioural consequences, and a deeper understanding of the mechanistic underpinnings of TMS will be critical for advancing it as a scientific and therapeutic tool.
Keywords: Mechanism of action; Motor cortex; Physiology; Transcranial magnetic stimulation.
Copyright © 2022 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Andrea Antal has received honoraria from NeuroCare GmbH, Germany and Savir GmbH, Germany. Peter T. Fox has received patents in multiple jurisdictions for various TMS technologies, including the cortical column cosine model (C3) and the delivery of transcranial magnetic stimulation in accordance with the C3 model in an image-guided or robotically controlled manner. These patents are assigned to the University of Texas Board of Regents and have been licensed for commercialization by the University of Texas to a private entity in which Dr. Fox has ownership interest. Mark Hallett is an inventor of patents held by NIH for an immunotoxin for the treatment of focal movement disorders and the H-coil for magnetic stimulation; in relation to the latter, he has received license fee payments from the NIH (from Brainsway). He is on the Medical Advisory Boards of CALA Health and Brainsway (both unpaid positions). He is on the Editorial Board of approximately 15 journals and receives royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, Springer, Wiley, Wolters Kluwer, and Elsevier. He has research grants from Medtronic, Inc. for a study of DBS for dystonia and CALA Health for studies of a device to suppress tremor. Hartwig R. Siebner has received honoraria as speaker from Sanofi Genzyme, Denmark and Novartis, Denmark, as consultant from Sanofi Genzyme, Denmark, Lophora, Denmark, and Lundbeck AS, Denmark, and as editor-in-chief (Neuroimage Clinical) and senior editor (NeuroImage) from Elsevier Publishers, Amsterdam, The Netherlands. He has received royalties as book editor from Springer Publishers, Stuttgart, Germany and from Gyldendal Publishers, Copenhagen, Denmark. Yoshikazu Ugawa has received honoraria from Takeda Pharmaceutical Company Limited, Eisai Co., Ltd., FP Pharmaceutical Corporation, Otsuka Pharmaceutical Co., Ltd., Elsevier Japan K. K., Kyowa Hakko Kirin Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, NIHON PHARMACEUTICAL Co., Ltd., and Novartis Pharma K.K. He has received royalties as journal editor from CHUGAI-IGAKUSHA, Igaku-Shoin Ltd, Medical View Co. Ltd., and Blackwell Publishing K.K. Walter Paulus has received honoraria as speaker from Philips, Medipark Clinic and as a consultant from Abott and Precisis AG. A.V. Peterchev has received research funding, travel support, patent royalties, consulting fees, equipment loans, hardware donations, and/or patent application support from Rogue Research, Tal Medical/Neurex, Magstim, MagVenture, Neuronetics, BTL Industries, and Advise Connect Inspire. Ulf Ziemann received grants from Janssen Pharmaceuticals NV and Takeda Pharmaceutical Company Ltd., and consulting fees from Bayer Vital GmbH, Pfizer GmbH and CorTec GmbH. All other authors have no conflict of interest to report.
Figures



Comment in
-
Facial nerve stimulation.Clin Neurophysiol. 2022 Dec;144:151. doi: 10.1016/j.clinph.2022.08.028. Epub 2022 Sep 24. Clin Neurophysiol. 2022. PMID: 36195511 No abstract available.
-
Reply to "Facial nerve stimulation".Clin Neurophysiol. 2022 Dec;144:152. doi: 10.1016/j.clinph.2022.09.008. Epub 2022 Sep 24. Clin Neurophysiol. 2022. PMID: 36195512 No abstract available.
References
-
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science 2007;317 (5846):1918–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

