Emerging structural insights into GPCR-β-arrestin interaction and functional outcomes
- PMID: 35738165
- PMCID: PMC7614528
- DOI: 10.1016/j.sbi.2022.102406
Emerging structural insights into GPCR-β-arrestin interaction and functional outcomes
Abstract
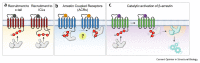
Agonist-induced recruitment of β-arrestins (βarrs) to G protein-coupled receptors (GPCRs) plays a central role in regulating the spatio-temporal aspects of GPCR signaling. Several recent studies have provided novel structural and functional insights into our understanding of GPCR-βarr interaction, subsequent βarr activation and resulting functional outcomes. In this review, we discuss these recent advances with a particular emphasis on recognition of receptor-bound phosphates by βarrs, the emerging concept of spatial positioning of key phosphorylation sites, the conformational transition in βarrs during partial to full-engagement, and structural differences driving functional outcomes of βarr isoforms. We also highlight the key directions that require further investigation going forward to fully understand the structural mechanisms driving βarr activation and functional responses.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

