Medicare Coverage and Out-of-Pocket Costs of Quadruple Drug Therapy for Heart Failure
- PMID: 35738713
- PMCID: PMC12293934
- DOI: 10.1016/j.jacc.2022.04.031
Medicare Coverage and Out-of-Pocket Costs of Quadruple Drug Therapy for Heart Failure
Abstract
Background: Beta-blockers, angiotensin receptor-neprilysin inhibitor (ARNI), mineralocorticoid receptor antagonists, and sodium-glucose cotransporter-2 inhibitors (SGLT2i), known as quadruple therapy, are recommended for patients with heart failure with reduced ejection fraction (HFrEF).
Objectives: This study sought to determine Medicare coverage and out-of-pocket (OOP) costs of quadruple therapy and regimens excluding ARNI or SGLT2i.
Methods: This study assessed cost sharing, prior authorization, and step therapy in all 4,068 Medicare prescription drug plans in 2020. OOP costs were determined during the standard coverage period and annually based on the Medicare Part D standard benefit, inclusive of deductible, standard coverage, coverage gap, and catastrophic coverage.
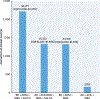
Results: Tier ≥3 cost sharing was required by 99.1% of plans for ARNI and 98.5% for at least 1 SGLT2i. Only ARNI required prior authorization (24.3% of plans), and step therapy was required only for SGLT2is (5.4%) and eplerenone (0.8%). The median 30-day standard coverage OOP cost of quadruple therapy was $94 (IQR: $84-$100), including $47 (IQR: $40-$47) for ARNI and $45 (IQR: $40-$47) for SGLT2i. The median annual OOP cost of quadruple therapy was $2,217 (IQR: $1,956-$2,579) compared with $1,319 (IQR: $1,067-$1,675) when excluding SGLT2i and $1,322 (IQR: $1,025-$1,588) when including SGLT2i and substituting an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker for ARNI. The median 30-day OOP cost of generic regimens was $3 (IQR: $0-$9).
Conclusions: Medicare drug plans restrict coverage of quadruple therapy through cost sharing, with OOP costs that are substantially higher than generic regimens. Quadruple therapy may be unaffordable for many Medicare patients with HFrEF unless medication prices and cost sharing are reduced.
Keywords: Medicare; drug costs; health insurance; heart failure.
Copyright © 2022 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Dr Faridi was supported by a grant from the National Institutes of Health (NIH) Clinical and Translational Science Award (KL2 TR001862), outside the scope of the present work. Dr Ross has been supported by the National Heart, Lung, and Blood Institute of the NIH (R01HS025164, R01HL144644), by the Agency for Healthcare Research and Quality (R01HS022882), and by the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International. Dr Dhruva has been supported by the National Heart, Lung, and Blood Institute of the NIH (K12HL138046), the National Evaluation System for Health Technology Coordinating Center, the Greenwall Foundation, and Arnold Ventures. Dr Ross has received research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the Medical Device Innovation Consortium as part of the National Evaluation System for Health Technology (NEST), from the Food and Drug Administration for the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), and from the Agency for Healthcare Research and Quality (R01HS022882); and he has been an expert witness at the request of Relator's attorneys, the Greene Law Firm, in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen Inc. Dr Dhruva has received honoraria from the Institute for Clinical and Economic Review as a member of the California Technology Assessment Forum. Dr Ahmad has received research funding from AstraZeneca, Boehringer Ingelheim, Amgen, and Cytokinetics. Dr Desai has worked under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures used for public reporting and pay for performance programs; and he has received research grants and/or been a consultant for Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, MyoKardia, Novartis, scPharmaceuticals, and Vifor Pharma. Dr Dayoub has reported that he has no relationships relevant to the contents of this paper to disclose.
Figures


Comment in
-
Value-Based Prices Could Establish Common Ground on Heart Failure Drug Pricing and Coverage.J Am Coll Cardiol. 2022 Jun 28;79(25):2526-2528. doi: 10.1016/j.jacc.2022.04.032. J Am Coll Cardiol. 2022. PMID: 35738714 No abstract available.
References
-
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. - PubMed
-
- Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. - PubMed
-
- Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117–1145. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

