Reduction in Ventricular Tachyarrhythmia Burden in Patients Enrolled in the RAID Trial
- PMID: 35738852
- PMCID: PMC9473303
- DOI: 10.1016/j.jacep.2022.02.018
Reduction in Ventricular Tachyarrhythmia Burden in Patients Enrolled in the RAID Trial
Abstract
Background: The RAID (Ranolazine Implantable Cardioverter-Defibrillator) randomized placebo-controlled trial showed that ranolazine treatment was associated with reduction in recurrent ventricular tachycardia (VT) requiring appropriate implantable cardioverter-defibrillator (ICD) therapy.
Objectives: This study aimed to identify groups of patients in whom ranolazine treatment would result in the highest reduction of ventricular tachyarrhythmia (VTA) burden.
Methods: Andersen-Gill analyses were performed to identify variables associated with risk for VTA burden among 1,012 patients enrolled in RAID. The primary endpoint was VTA burden defined as VTA episodes requiring appropriate treatment.
Results: Multivariate analysis identified 7 factors associated with increased VTA burden: history of VTA, age ≥65 years, New York Heart Association functional class ≥III, QRS complex (≥130 ms), low ejection fraction (<30%), atrial fibrillation (AF), and concomitant antiarrhythmic drug (AAD) therapy. The effect of ranolazine on VTA burden was seen among patients without concomitant AAD therapy (HR [HR]: 0.68; 95% CI: 0.55-0.84; P < 0.001), whereas no effect was seen among those who are concomitantly treated with other AADs (HR: 1.33; 95% CI: 0.90-1.96; P = 0.16); P = 0.003 for interaction. In patients with cardiac resynchronization therapy (CRT) ICDs, ranolazine treatment was associated with a 36% risk reduction for VTA recurrence (HR: 0.64; 95% CI: 0.47-0.86; P < 0.001), whereas among patients with ICDs without CRT no significant effect was noted (HR: 0.94; 95% CI: 0.74-1.18; P = 0.57); P = 0.047 for interaction.
Conclusions: In patients with high risk for VTA, ranolazine is effective in reducing VTA burden, with significantly greater effect in CRT-treated patients, those without AF, and those not treated with concomitant AADs. In patients already on AADs or those with AF, the addition of ranolazine did not affect VTA burden. (Ranolazine Implantable Cardioverter-Defibrillator Trial [RAID]; NCT01215253).
Keywords: ICD; arrhythmia; cardioverter-defibrillator; ranolazine; ventricular tachycardia.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This study was supported by grants from the National Heart, Lung, and Blood Institute: Clinical Coordination Center (UO1 HL096607) and Data Coordination Center (UO1 HL096610. Study drug and additional financial support for drug distribution was provided by Gilead Sciences grant number IN-US-259-0125. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

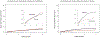
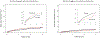
Comment in
-
A Role for Ranolazine in the Treatment of Ventricular Arrhythmias?JACC Clin Electrophysiol. 2022 Jun;8(6):763-765. doi: 10.1016/j.jacep.2022.04.010. JACC Clin Electrophysiol. 2022. PMID: 35738853 No abstract available.
References
-
- Schron EB, Exner DV, Yao Q, Jenkins LS, Steinberg JS, Cook JR, Kutalek SP, Friedman PL, Bubien RS, Page RL and Powell J. Quality of life in the antiarrhythmics versus implantable defibrillators trial: Impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105:589–594. - PubMed
-
- Godemann F, Butter C, Lampe F, Linden M, Werner S and Behrens S. Determinants of the quality of life (QoL) in patients with an implantable cardioverter/defibrillator (ICD). Quality of Life Research. 2004;13:411–416. - PubMed
-
- Larsen GK, Evans J, Lambert WE, Chen Y and Raitt MH. Shocks burden and increased mortality in implantable cardioverter-defibrillator patients. Heart Rhythm. 2011;8:1881–6. - PubMed
-
- Thireau J, Pasquié J-L, Martel E, Le Guennec J-Y and Richard S. New drugs vs. old concepts: A fresh look at antiarrhythmics. Pharmacology & Therapeutics. 2011;132:125–145. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

