E-cigarettes to Augment Stop Smoking In-person Support and Treatment With Varenicline (E-ASSIST): A Pragmatic Randomized Controlled Trial
- PMID: 35738868
- PMCID: PMC9384384
- DOI: 10.1093/ntr/ntac149
E-cigarettes to Augment Stop Smoking In-person Support and Treatment With Varenicline (E-ASSIST): A Pragmatic Randomized Controlled Trial
Abstract
Aim: To examine whether, in adults receiving behavioral support, offering e-cigarettes together with varenicline helps more people stop smoking cigarettes than varenicline alone.
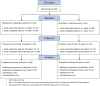
Methods: A two-group, parallel arm, pragmatic randomized controlled trial was conducted in six English stop smoking services from 2019-2020. Adults enrolled onto a 12-week programme of in-person one-to-one behavioral smoking cessation support (N = 92) were randomized to receive either (1) a nicotine e-cigarette starter kit alongside varenicline or (2) varenicline alone. The primary outcome was biochemically verified abstinence from cigarette smoking between weeks 9-to-12 post quit date, with those lost to follow-up considered not abstinent. The trial was stopped early due to COVID-19 restrictions and a varenicline recall (92/1266 participants used).
Results: Nine-to-12-week smoking abstinence rates were 47.9% (23/48) in the e-cigarette-varenicline group compared with 31.8% (14/44) in the varenicline-only group, a 51% increase in abstinence among those offered e-cigarettes; however, the confidence interval (CI) was wide, including the possibility of no difference (risk ratio [RR] = 1.51, 95% CI = 0.91-2.64). The e-cigarette-varenicline group had 43% lower hazards of relapse from continuous abstinence than the varenicline-only group (hazards ratio [HR] = 0.57, 95% CI = 0.34-0.96). Attendance for 12 weeks was higher in the e-cigarette-varenicline than varenicline-only group (54.2% vs. 36.4%; RR = 1.49, 95% CI = 0.95-2.47), but similar proportions of participants in both groups used varenicline daily for ≥8 weeks after quitting (22.9% versus 22.7%; RR = 1.01, 95% CI = 0.47-2.20). Estimates were too imprecise to determine how adverse events differed by group.
Conclusion: Tentative evidence suggests that offering e-cigarettes alongside varenicline to people receiving behavioral support may be more effective for smoking cessation than varenicline alone.
Implications: Offering e-cigarettes to people quitting smoking with varenicline may help them remain abstinent from cigarettes, but the evidence is tentative because our sample size was smaller than planned-caused by Coronavirus Disease 2019 (COVID-19) restrictions and a manufacturing recall. This meant our effect estimates were imprecise, and additional evidence is needed to confirm that providing e-cigarettes and varenicline together helps more people remain abstinent than varenicline alone.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco.
Figures

References
-
- Drope J, Schluger NW.. The Tobacco Atlas. 6th ed. Atlanta, GA: American Cancer Society and Vital Strategies; 2018.
-
- Raupach T, West R, Brown J.. The most “successful” method for failing to quit smoking is unassisted cessation. Nicotine Tob Res. 2013;15(3):748–749. - PubMed
-
- Anthenelli RM, Benowitz NL, West R, et al. . Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. - PubMed
-
- Ferguson J, Bauld L, Chesterman J, Judge K.. The English smoking treatment services: one-year outcomes. Addiction. 2005;100(Suppl 2):59–69. - PubMed
-
- E-cigarette (ADDICTO:0000212). In: AddictO Vocab. AddictO Vocab; 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

