89Zr-Labeled High-Density Lipoprotein Nanoparticle PET Imaging Reveals Tumor Uptake in Patients with Esophageal Cancer
- PMID: 35738904
- PMCID: PMC9730913
- DOI: 10.2967/jnumed.121.263330
89Zr-Labeled High-Density Lipoprotein Nanoparticle PET Imaging Reveals Tumor Uptake in Patients with Esophageal Cancer
Abstract
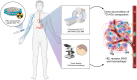
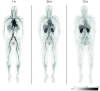
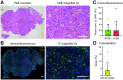
Nanomedicine holds promise for the delivery of therapeutic and imaging agents to improve cancer treatment outcomes. Preclinical studies have demonstrated that high-density lipoprotein (HDL) nanoparticles accumulate in tumor tissue on intravenous administration. Whether this HDL-based nanomedicine concept is feasible in patients is unexplored. Using a multimodal imaging approach, we aimed to assess tumor uptake of exogenously administered HDL nanoparticles in patients with esophageal cancer. Methods: The HDL mimetic CER-001 was radiolabeled using 89Zr to allow for PET/CT imaging. Patients with primary esophageal cancer staged T2 and above were recruited for serial 89Zr-HDL PET/CT imaging before starting chemoradiation therapy. In addition, patients underwent routine 18F-FDG PET/CT and 3-T MRI scanning (diffusion-weighted imaging/intravoxel incoherent motion imaging and dynamic contrast-enhanced MRI) to assess tumor glucose metabolism, tumor cellularity and microcirculation perfusion, and tumor vascular permeability. Tumor biopsies were analyzed for the expression of HDL scavenger receptor class B1 and macrophage marker CD68 using immunofluorescence staining. Results: Nine patients with adenocarcinoma or squamous cell carcinoma underwent all study procedures. After injection of 89Zr-HDL (39.2 ± 1.2 [mean ± SD] MBq), blood-pool SUVmean decreased over time (11.0 ± 1.7, 6.5 ± 0.6, and 3.3 ± 0.5 at 1, 24, and 72 h, respectively), whereas liver and spleen SUVmean remained relatively constant (4.1 ± 0.6, 4.0 ± 0.8, and 4.3 ± 0.8 at 1, 24, and 72 h, respectively, for the liver; 4.1 ± 0.3, 3.4 ± 0.3, and 3.1 ± 0.4 at 1, 24, and 72 h, respectively, for the spleen) and kidney SUVmean markedly increased over time (4.1 ± 0.9, 9.3 ± 1.4, and 9.6 ± 2.0 at 1, 24, and 72 h, respectively). Tumor uptake (SUVpeak) increased over time (3.5 ± 1.1 and 5.5 ± 2.1 at 1 and 24 h, respectively [P = 0.016]; 5.7 ± 1.4 at 72 h [P = 0.001]). The effective dose of 89Zr-HDL was 0.523 ± 0.040 mSv/MBq. No adverse events were observed after the administration of 89Zr-HDL. PET/CT and 3-T MRI measures of tumor glucose metabolism, tumor cellularity and microcirculation perfusion, and tumor vascular permeability did not correlate with tumor uptake of 89Zr-HDL, suggesting that a specific mechanism mediated the accumulation of 89Zr-HDL. Immunofluorescence staining of clinical biopsies demonstrated scavenger receptor class B1 and CD68 positivity in tumor tissue, establishing a potential cellular mechanism of action. Conclusion: To our knowledge, this was the first 89Zr-HDL study in human oncology. 89Zr-HDL PET/CT imaging demonstrated that intravenously administered HDL nanoparticles accumulated in tumors of patients with esophageal cancer. The administration of 89Zr-HDL was safe. These findings may support the development of HDL nanoparticles as a clinical delivery platform for drug agents. 89Zr-HDL imaging may guide drug development and serve as a biomarker for individualized therapy.
Keywords: PET/CT; esophageal cancer; high-density lipoprotein; nanomedicine; zirconium.
© 2022 by the Society of Nuclear Medicine and Molecular Imaging.
Figures


Similar articles
-
PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles.J Nucl Med. 2015 Aug;56(8):1272-7. doi: 10.2967/jnumed.115.158956. Epub 2015 Jun 25. J Nucl Med. 2015. PMID: 26112022 Free PMC article.
-
HDL mimetic CER-001 targets atherosclerotic plaques in patients.Atherosclerosis. 2016 Aug;251:381-388. doi: 10.1016/j.atherosclerosis.2016.05.038. Epub 2016 May 27. Atherosclerosis. 2016. PMID: 27263077
-
In Vivo PET Imaging of HDL in Multiple Atherosclerosis Models.JACC Cardiovasc Imaging. 2016 Aug;9(8):950-61. doi: 10.1016/j.jcmg.2016.01.020. Epub 2016 May 25. JACC Cardiovasc Imaging. 2016. PMID: 27236528 Free PMC article.
-
Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials?Front Pharmacol. 2016 May 24;7:131. doi: 10.3389/fphar.2016.00131. eCollection 2016. Front Pharmacol. 2016. PMID: 27252651 Free PMC article. Review.
-
High-Density Lipoprotein Nanobiologics for Precision Medicine.Acc Chem Res. 2018 Jan 16;51(1):127-137. doi: 10.1021/acs.accounts.7b00339. Epub 2017 Dec 27. Acc Chem Res. 2018. PMID: 29281244 Free PMC article. Review.
Cited by
-
Exploring innovative strides in radiolabeled nanoparticle progress for multimodality cancer imaging and theranostic applications.Cancer Imaging. 2024 Sep 20;24(1):127. doi: 10.1186/s40644-024-00762-z. Cancer Imaging. 2024. PMID: 39304961 Free PMC article. Review.
-
Nanoscale Radiotheranostics for Cancer Treatment: From Bench to Bedside.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024 Sep-Oct;16(5):e2006. doi: 10.1002/wnan.2006. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024. PMID: 39407431 Free PMC article. Review.
-
Good practices for 89Zr radiopharmaceutical production and quality control.EJNMMI Radiopharm Chem. 2024 May 11;9(1):40. doi: 10.1186/s41181-024-00258-y. EJNMMI Radiopharm Chem. 2024. PMID: 38733556 Free PMC article. Review.
-
Radiolabeling lipoproteins to study and manage disease.Eur J Nucl Med Mol Imaging. 2025 Apr 28:10.1007/s00259-025-07281-4. doi: 10.1007/s00259-025-07281-4. Online ahead of print. Eur J Nucl Med Mol Imaging. 2025. PMID: 40293448 Free PMC article. Review.
References
-
- van Putten M, de Vos-Geelen J, Nieuwenhuijzen GAP, et al. . Long-term survival improvement in oesophageal cancer in the Netherlands. Eur J Cancer. 2018;94:138–147. - PubMed
-
- Shah MA, Kennedy EB, Catenacci DV, et al. . Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol. 2020;38:2677–2694. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
