Secretion of the siderophore rhizoferrin is regulated by the cAMP-PKA pathway and is involved in the virulence of Mucor lusitanicus
- PMID: 35739200
- PMCID: PMC9226013
- DOI: 10.1038/s41598-022-14515-0
Secretion of the siderophore rhizoferrin is regulated by the cAMP-PKA pathway and is involved in the virulence of Mucor lusitanicus
Abstract
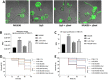
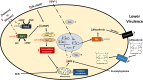
Mucormycosis is a fungal infection caused by Mucorales, with a high mortality rate. However, only a few virulence factors have been described in these organisms. This study showed that deletion of rfs, which encodes the enzyme for the biosynthesis of rhizoferrin, a siderophore, in Mucor lusitanicus, led to a lower virulence in diabetic mice and nematodes. Upregulation of rfs correlated with the increased toxicity of the cell-free supernatants of the culture broth (SS) obtained under growing conditions that favor oxidative metabolism, such as low glucose levels or the presence of H2O2 in the culture, suggesting that oxidative metabolism enhances virulence through rhizoferrin production. Meanwhile, growing M. lusitanicus in the presence of potassium cyanide, N-acetylcysteine, a higher concentration of glucose, or exogenous cAMP, or the deletion of the gene encoding the regulatory subunit of PKA (pkaR1), correlated with a decrease in the toxicity of SS, downregulation of rfs, and reduction in rhizoferrin production. These observations indicate the involvement of the cAMP-PKA pathway in the regulation of rhizoferrin production and virulence in M. lusitanicus. Moreover, rfs upregulation was observed upon macrophage interaction or during infection with spores in mice, suggesting a pivotal role of rfs in M. lusitanicus infection.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Morin-Sardin S, Nodet P, Coton E, Jany JL. Mucor: A Janus-faced fungal genus with human health impact and industrial applications. Fungal Biol. Rev. 2017;31:12–32. doi: 10.1016/j.fbr.2016.11.002. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

