Elucidating macular structure-function correlations in glaucoma
- PMID: 35739208
- PMCID: PMC9226060
- DOI: 10.1038/s41598-022-13730-z
Elucidating macular structure-function correlations in glaucoma
Abstract
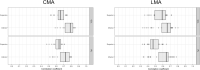
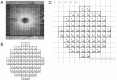
Correlation between structural data from optical coherence tomography and functional data from the visual field may be suboptimal because of poor mapping of OCT measurement locations to VF stimuli. We tested the hypothesis that stronger structure-function correlations in the macula can be achieved with fundus-tracking perimetery, by precisely mapping OCT measurements to VF sensitivity at the same location. The conventional 64 superpixel (3° × 3°) OCT grid was mapped to VF sensitivities averaged in 40 corresponding VF units with standard automated perimetry (conventional mapped approach, CMA) in 38 glaucoma patients and 10 healthy subjects. Similarly, a 144 superpixel (2° × 2°) OCT grid was mapped to each of the 68 locations with fundus-tracking perimetry (localized mapped approach, LMA). For each approach, the correlation between sensitivity at each VF unit and OCT superpixel was computed. Vector maps showing the maximum correlation between each VF unit and OCT pixel was generated. CMA yielded significantly higher structure-function correlations compared to LMA. Only 20% of the vectors with CMA and < 5% with LMA were within corresponding mapped OCT superpixels, while most were directed towards loci with structural damage. Measurement variability and patterns of structural damage more likely impact correlations compared to precise mapping of VF stimuli.
© 2022. The Author(s).
Conflict of interest statement
B.C.C. is consultant for CenterVue and Heidelberg Engineering. SG, GPS, OD, PER, LMS, MTN and JRV do not have competing interests.
Figures
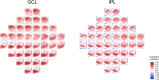
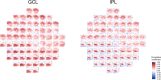
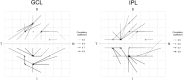
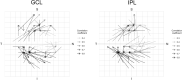


References
-
- Nouri-Mahdavi K, et al. Macular ganglion cell/inner plexiform layer measurements by spectral domain optical coherence tomography for detection of early glaucoma and comparison to retinal nerve fiber layer measurements. Am. J. Ophthalmol. 2013;156:1297–1307.e2. doi: 10.1016/j.ajo.2013.08.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

