Pathological and neurophysiological outcomes of seeding human-derived tau pathology in the APP-KI NL-G-F and NL-NL mouse models of Alzheimer's Disease
- PMID: 35739575
- PMCID: PMC9219251
- DOI: 10.1186/s40478-022-01393-w
Pathological and neurophysiological outcomes of seeding human-derived tau pathology in the APP-KI NL-G-F and NL-NL mouse models of Alzheimer's Disease
Abstract
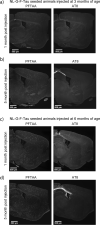
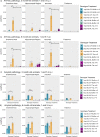
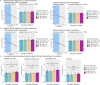
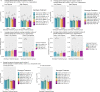
The two main histopathological hallmarks that characterize Alzheimer's Disease are the presence of amyloid plaques and neurofibrillary tangles. One of the current approaches to studying the consequences of amyloid pathology relies on the usage of transgenic animal models that incorporate the mutant humanized form of the amyloid precursor protein (hAPP), with animal models progressively developing amyloid pathology as they age. However, these mice models generally overexpress the hAPP protein to facilitate the development of amyloid pathology, which has been suggested to elicit pathological and neuropathological changes unrelated to amyloid pathology. In this current study, we characterized APP knock-in (APP-KI) animals, that do not overexpress hAPP but still develop amyloid pathology to understand the influence of protein overexpression. We also induced tau pathology via human-derived tau seeding material to understand the neurophysiological effects of amyloid and tau pathology. We report that tau-seeded APP-KI animals progressively develop tau pathology, exacerbated by the presence of amyloid pathology. Interestingly, older amyloid-bearing, tau-seeded animals exhibited more amyloid pathology in the entorhinal area, isocortex and hippocampus, but not thalamus, which appeared to correlate with impairments in gamma oscillations before seeding. Tau-seeded animals also featured immediate deficits in power spectra values and phase-amplitude indices in the hippocampus after seeding, with gamma power spectra deficits persisting in younger animals. Both deficits in hippocampal phase-amplitude coupling and gamma power differentiate tau-seeded, amyloid-positive animals from buffer controls. Based on our results, impairments in gamma oscillations appear to be strongly associated with the presence and development of amyloid and tau pathology, and may also be an indicator of neuropathology, network dysfunction, and even potential disposition to the future development of amyloid pathology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they are working for Janssen Pharmaceutica NV. The authors declare no other commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. - PubMed
-
- Yoshiyama Y, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

