Altered Mitochondrial Opa1-Related Fusion in Mouse Promotes Endothelial Cell Dysfunction and Atherosclerosis
- PMID: 35739974
- PMCID: PMC9219969
- DOI: 10.3390/antiox11061078
Altered Mitochondrial Opa1-Related Fusion in Mouse Promotes Endothelial Cell Dysfunction and Atherosclerosis
Abstract
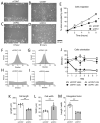
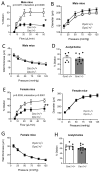
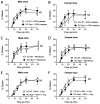
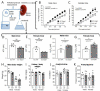
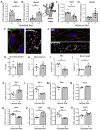
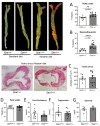
Flow (shear stress)-mediated dilation (FMD) of resistance arteries is a rapid endothelial response involved in tissue perfusion. FMD is reduced early in cardiovascular diseases, generating a major risk factor for atherosclerosis. As alteration of mitochondrial fusion reduces endothelial cells' (ECs) sprouting and angiogenesis, we investigated its role in ECs responses to flow. Opa1 silencing reduced ECs (HUVECs) migration and flow-mediated elongation. In isolated perfused resistance arteries, FMD was reduced in Opa1+/- mice, a model of the human disease due to Opa1 haplo-insufficiency, and in mice with an EC specific Opa1 knock-out (EC-Opa1). Reducing mitochondrial oxidative stress restored FMD in EC-Opa1 mice. In isolated perfused kidneys from EC-Opa1 mice, flow induced a greater pressure, less ATP, and more H2O2 production, compared to control mice. Opa1 expression and mitochondrial length were reduced in ECs submitted in vitro to disturbed flow and in vivo in the atheroprone zone of the mouse aortic cross. Aortic lipid deposition was greater in Ldlr-/--Opa1+/- and in Ldlr-/--EC-Opa1 mice than in control mice fed with a high-fat diet. In conclusion, we found that reduction in mitochondrial fusion in mouse ECs altered the dilator response to shear stress due to excessive superoxide production and induced greater atherosclerosis development.
Keywords: arteries; atherosclerosis; blood flow; endothelial cell; mitochondrial fusion; shear stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

