Evolutionary Adaptations of Parasitic Flatworms to Different Oxygen Tensions
- PMID: 35739999
- PMCID: PMC9220675
- DOI: 10.3390/antiox11061102
Evolutionary Adaptations of Parasitic Flatworms to Different Oxygen Tensions
Abstract
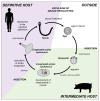
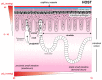
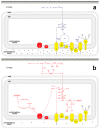
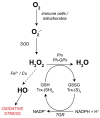
During the evolution of the Earth, the increase in the atmospheric concentration of oxygen gave rise to the development of organisms with aerobic metabolism, which utilized this molecule as the ultimate electron acceptor, whereas other organisms maintained an anaerobic metabolism. Platyhelminthes exhibit both aerobic and anaerobic metabolism depending on the availability of oxygen in their environment and/or due to differential oxygen tensions during certain stages of their life cycle. As these organisms do not have a circulatory system, gas exchange occurs by the passive diffusion through their body wall. Consequently, the flatworms developed several adaptations related to the oxygen gradient that is established between the aerobic tegument and the cellular parenchyma that is mostly anaerobic. Because of the aerobic metabolism, hydrogen peroxide (H2O2) is produced in abundance. Catalase usually scavenges H2O2 in mammals; however, this enzyme is absent in parasitic platyhelminths. Thus, the architecture of the antioxidant systems is different, depending primarily on the superoxide dismutase, glutathione peroxidase, and peroxiredoxin enzymes represented mainly in the tegument. Here, we discuss the adaptations that parasitic flatworms have developed to be able to transit from the different metabolic conditions to those they are exposed to during their life cycle.
Keywords: Cestoda; anaerobic metabolism; mitochondria; oxygen tension; platyhelminthes; tegument.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Nutritional adaptations to parasitism within the platyhelminthes.Int J Parasitol. 1997 Jun;27(6):693-704. doi: 10.1016/s0020-7519(97)00011-8. Int J Parasitol. 1997. PMID: 9229252 Review.
-
Superoxide anion and hydrogen peroxide metabolism in soybean embryonic axes during germination.Biochim Biophys Acta. 1991 Jul 8;1074(2):277-83. doi: 10.1016/0304-4165(91)90164-c. Biochim Biophys Acta. 1991. PMID: 1648400
-
[The influence of Janicki cercomer theory on the development of platyhelminthes systematics and evolution investigations].Wiad Parazytol. 2005;51(4):345-58. Wiad Parazytol. 2005. PMID: 16913510 Review. Polish.
-
Biochemistry and Physiology of Reactive Oxygen Species in Euglena.Adv Exp Med Biol. 2017;979:47-64. doi: 10.1007/978-3-319-54910-1_4. Adv Exp Med Biol. 2017. PMID: 28429317 Review.
-
Enhanced oxygen delivery reverses anaerobic metabolic states in prolonged sandwich rat hepatocyte culture.Exp Cell Res. 1999 Jan 10;246(1):221-32. doi: 10.1006/excr.1998.4295. Exp Cell Res. 1999. PMID: 9882531
Cited by
-
Insights into the Role of Oxidative Stress and Reactive Oxygen Species in Parasitic Diseases.Antioxidants (Basel). 2023 Apr 27;12(5):1010. doi: 10.3390/antiox12051010. Antioxidants (Basel). 2023. PMID: 37237875 Free PMC article.
-
Hooked on fish blood: the reliance of a gill parasite on haematophagy.Proc Biol Sci. 2024 Oct;291(2033):20241611. doi: 10.1098/rspb.2024.1611. Epub 2024 Oct 30. Proc Biol Sci. 2024. PMID: 39474874 Free PMC article.
-
Littorina snails and Microphallus trematodes: Diverse consequences of the trematode-induced metabolic shifts.Parasitol Res. 2024 May 31;123(6):229. doi: 10.1007/s00436-024-08244-8. Parasitol Res. 2024. PMID: 38819740
-
Adaptive evolution of stress response genes in parasites aligns with host niche diversity.BMC Biol. 2025 Jan 13;23(1):10. doi: 10.1186/s12915-024-02091-w. BMC Biol. 2025. PMID: 39800686 Free PMC article.
-
Transcriptome changes of liver fluke Opisthorchis viverrini in diabetic hamsters.Parasite. 2024;31:54. doi: 10.1051/parasite/2024056. Epub 2024 Sep 13. Parasite. 2024. PMID: 39269256 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

