Antioxidant Effect of Tyr-Ala Extracted from Zein on INS-1 Cells and Type 2 Diabetes High-Fat-Diet-Induced Mice
- PMID: 35740008
- PMCID: PMC9219942
- DOI: 10.3390/antiox11061111
Antioxidant Effect of Tyr-Ala Extracted from Zein on INS-1 Cells and Type 2 Diabetes High-Fat-Diet-Induced Mice
Abstract
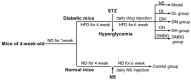
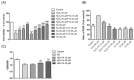
Type 2 diabetes mellitus (T2DM) is associated with an oxidative milieu that often leads to adverse health problems. Bioactive peptides of zein possess outstanding antioxidant activity; however, their effects on hyperglycemia-related oxidative stress remain elusive. In the present study, the dipeptide Tyr-Ala (YA), a functional peptide with typical health benefits, was applied to alleviate oxidative stress in pancreatic islets under hyperglycemic conditions. By detecting viability, antioxidant ability, and insulin secretion in INS-1 cells, YA showed excellent protection of INS-1 cells from H2O2 oxidative stress, erasing reactive oxygen species (ROS) and promoting insulin secretion. Moreover, by Western blotting, we found that YA can regulate the PI3K/Akt signaling pathway associated with glycometabolism. After establishing a T2DM mice model, we treated mice with YA and measured glucose, insulin, hemoglobin A1C (HbA1c), total cholesterol (TC), triglyceride (TG), and malonaldehyde (MDA) levels and activities of superoxide dismutase (SOD) and glutathione (GSH) from blood samples. We observed that YA could reduce the production of glucose, insulin, HbA1c, TC, TG, and MDA, in addition to enhancing the activities of SOD and GSH. YA could also repair the function of the kidneys and pancreas of T2DM mice. Along with the decline in fasting blood glucose, the oxidative stress in islets was alleviated in T2DM mice after YA administration. This may improve the health situation of diabetic patients in the future.
Keywords: INS-1 cells; peptide; reactive oxygen species; type 2 diabetes; tyr-ala.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



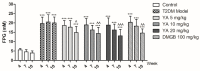
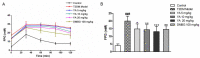


Similar articles
-
Food-derived cyanidin-3-O-glucoside alleviates oxidative stress: evidence from the islet cell line and diabetic db/db mice.Food Funct. 2021 Nov 15;12(22):11599-11610. doi: 10.1039/d1fo02385c. Food Funct. 2021. PMID: 34713882
-
Uncoupling protein-2 mediates the protective action of berberine against oxidative stress in rat insulinoma INS-1E cells and in diabetic mouse islets.Br J Pharmacol. 2014 Jul;171(13):3246-54. doi: 10.1111/bph.12666. Br J Pharmacol. 2014. PMID: 24588674 Free PMC article.
-
[γ-aminobutyric acid fortified rice alleviated oxidative stress and pancreatic injury in type 2 diabetic mice].Wei Sheng Yan Jiu. 2019 Mar;48(2):179-199. Wei Sheng Yan Jiu. 2019. PMID: 31133092 Chinese.
-
Antidiabetic effect of total flavonoids from Sanguis draxonis in type 2 diabetic rats.J Ethnopharmacol. 2013 Oct 7;149(3):729-36. doi: 10.1016/j.jep.2013.07.035. Epub 2013 Aug 7. J Ethnopharmacol. 2013. PMID: 23933499
-
Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench) Improves Antioxidant Capacity via PI3K/AKT Pathways and Nrf2 Translocation in a Type 2 Diabetes Model.Molecules. 2019 May 17;24(10):1906. doi: 10.3390/molecules24101906. Molecules. 2019. PMID: 31108940 Free PMC article.
Cited by
-
Functional profiling of synthetic camel milk-derived peptides with implication in glucose transport and diabetes.PLoS One. 2025 Mar 28;20(3):e0320812. doi: 10.1371/journal.pone.0320812. eCollection 2025. PLoS One. 2025. PMID: 40153398 Free PMC article.
References
-
- Juarez-Flores D.L., Ezquerra M., Gonzalez-Casacuberta I., Ormazabal A., Moren C., Tolosa E., Fucho R., Guitart-Mampel M., Casado M., Valldeoriola F., et al. Disrupted Mitochondrial and Metabolic Plasticity Underlie Comorbidity between Age-Related and Degenerative Disorders as Parkinson Disease and Type 2 Diabetes Mellitus. Antioxidants. 2020;9:1063. doi: 10.3390/antiox9111063. - DOI - PMC - PubMed
-
- Etsassala N.G.E.R., Badmus J.A., Marnewick J.L., Egieyeh S., Iwuoha E.I., Nchu F., Hussein A.A. Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities, Molecular Docking, and Antioxidant Capacities of Plectranthus ecklonii Constituents. Antioxidants. 2022;11:378. doi: 10.3390/antiox11020378. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

