A Multifactorial Regulation of Glutathione Metabolism behind Salt Tolerance in Rice
- PMID: 35740011
- PMCID: PMC9219684
- DOI: 10.3390/antiox11061114
A Multifactorial Regulation of Glutathione Metabolism behind Salt Tolerance in Rice
Abstract
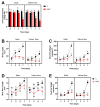
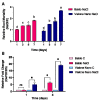
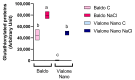
Knowledge of the stress-induced metabolic alterations in tolerant and sensitive plants is pivotal for identifying interesting traits that improve plant resilience toward unfavorable environmental conditions. This represents a hot topic area of plant science, particularly for crops, due to its implication in food security. Two rice varieties showing dissimilar resistance to salt, Baldo and Vialone Nano, have been studied to investigate the mechanisms underpinning tolerance toward salinity, and these studies have focused on the root system. A detailed analysis of the salt stress-dependent modulation of the redox network is here presented. The different phenotype observed after salt exposure in the two rice varieties is coherent with a differential regulation of cell-cycle progression and cell-death patterns observed at root level. Baldo, the tolerant variety, already showed a highly responsive antioxidative capacity in control conditions. Consistently, stressed Baldo plants showed a different pattern of H2O2 accumulation compared to Vialone Nano. Moreover, glutathione metabolism was finely modulated at transcriptional, post-transcriptional, and post-translational levels in Baldo. These results contribute to highlight the role of ROS and antioxidative pathways as a part of a complex redox network activated in rice toward salt stress.
Keywords: Oryza sativa; abiotic stress; cell cycle; glutathione metabolism; hydrogen peroxide; miRNA395; rice; salt stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Differential modulation of photosynthesis, ROS and antioxidant enzyme activities in stress-sensitive and -tolerant rice cultivars during salinity and drought upon restriction of COX and AOX pathways of mitochondrial oxidative electron transport.J Plant Physiol. 2022 Jan;268:153583. doi: 10.1016/j.jplph.2021.153583. Epub 2021 Nov 29. J Plant Physiol. 2022. PMID: 34871988
-
Fast Regulation of Hormone Metabolism Contributes to Salt Tolerance in Rice (Oryzasativa spp. Japonica, L.) by Inducing Specific Morpho-Physiological Responses.Plants (Basel). 2018 Sep 15;7(3):75. doi: 10.3390/plants7030075. Plants (Basel). 2018. PMID: 30223560 Free PMC article.
-
Modulation in growth, biochemical attributes and proteome profile of rice cultivars under salt stress.Plant Physiol Biochem. 2020 Jan;146:55-70. doi: 10.1016/j.plaphy.2019.11.011. Epub 2019 Nov 7. Plant Physiol Biochem. 2020. PMID: 31733605
-
Advances and Challenges in the Breeding of Salt-Tolerant Rice.Int J Mol Sci. 2020 Nov 9;21(21):8385. doi: 10.3390/ijms21218385. Int J Mol Sci. 2020. PMID: 33182265 Free PMC article. Review.
-
Advances in Sensing, Response and Regulation Mechanism of Salt Tolerance in Rice.Int J Mol Sci. 2021 Feb 24;22(5):2254. doi: 10.3390/ijms22052254. Int J Mol Sci. 2021. PMID: 33668247 Free PMC article. Review.
Cited by
-
Deciphering the Genetic Mechanisms of Salt Tolerance in Sorghum bicolor L.: Key Genes and SNP Associations from Comparative Transcriptomic Analyses.Plants (Basel). 2023 Jul 13;12(14):2639. doi: 10.3390/plants12142639. Plants (Basel). 2023. PMID: 37514252 Free PMC article.
-
Exploring Natural Variations in Arabidopsis thaliana: Plant Adaptability to Salt Stress.Plants (Basel). 2024 Apr 10;13(8):1069. doi: 10.3390/plants13081069. Plants (Basel). 2024. PMID: 38674478 Free PMC article.
-
Transcriptomic and metabolomic analyses reveal the positive effect of moderate concentration of sodium chloride treatment on the production of β-carotene, torulene, and torularhodin in oleaginous red yeast Rhodosporidiobolus odoratus XQR.Food Chem (Oxf). 2024 Aug 30;9:100221. doi: 10.1016/j.fochms.2024.100221. eCollection 2024 Dec 30. Food Chem (Oxf). 2024. PMID: 39399738 Free PMC article.
-
Transcriptional Landscape of Cotton Roots in Response to Salt Stress at Single-cell Resolution.Plant Commun. 2023 Oct 27;5(2):100740. doi: 10.1016/j.xplc.2023.100740. Online ahead of print. Plant Commun. 2023. PMID: 39492159 Free PMC article.
-
Effects of Exogenous (K+) Potassium Application on Plant Hormones in the Roots of Tamarix ramosissima under NaCl Stress.Genes (Basel). 2022 Oct 6;13(10):1803. doi: 10.3390/genes13101803. Genes (Basel). 2022. PMID: 36292689 Free PMC article.
References
-
- Zaki Mostafa Ali F. The Determinants of Salinity Tolerance in Maize (Zea Mays L.) University of Groningen; Groningen, The Netherlands: 2011.
-
- Safdar H., Amin A., Shafiq Y., Ali A., Yasin R., Sarwar M.I. Abbas Shoukat, Maqsood Ul Hussan, Muhammad Ishtiaq Sarwar. A Review: Impact of Salinity on Plant Growth. Nat. Sci. 2019;1:34–40. doi: 10.7537/marsnsj170119.06. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

