Antioxidant Properties of Cerium Oxide Nanoparticles Prevent Retinal Neovascular Alterations In Vitro and In Vivo
- PMID: 35740031
- PMCID: PMC9220105
- DOI: 10.3390/antiox11061133
Antioxidant Properties of Cerium Oxide Nanoparticles Prevent Retinal Neovascular Alterations In Vitro and In Vivo
Abstract
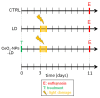
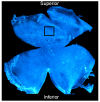
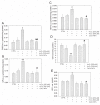
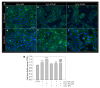
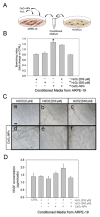
In this study, we investigated whether cerium oxide nanoparticles (CeO2-NPs), a promising antioxidant nanomaterial, may contrast retinal vascular alterations induced by oxidative damage in vitro and in vivo. For the in vivo experiments, the light damage (LD) animal model of Age-Related Macular Degeneration (AMD) was used and the CeO2-NPs were intravitreally injected. CeO2-NPs significantly decreased vascular endothelial growth factor (VEGF) protein levels, reduced neovascularization in the deep retinal plexus, and inhibited choroidal sprouting into the photoreceptor layer. The in vitro experiments were performed on human retinal pigment epithelial (ARPE-19) cells challenged with H2O2; we demonstrated that CeO2-NPs reverted H2O2-induced oxidative stress-dependent effects on this cell model. We further investigated the RPE-endothelial cells interaction under oxidative stress conditions in the presence or absence of CeO2-NPs through two experimental paradigms: (i) treatment of human umbilical vein endothelial cells (HUVECs) with conditioned media from ARPE-19 cells, and (ii) coculture of ARPE-19 and HUVECs. In both experimental conditions, CeO2-NPs were able to revert the detrimental effect of H2O2 on angiogenesis in vitro by realigning the level of tubule formation to that of the control. Altogether, our results indicate, for the first time, that CeO2-NPs can counteract retinal neovascularization and may be a new therapeutic strategy for the treatment of wet AMD.
Keywords: ARPE-19; HUVEC; RPE; VEGF; angiogenesis; cerium oxide nanoparticles; glycative stress; oxidative stress; wet AMD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Nanoceria Particles Are an Eligible Candidate to Prevent Age-Related Macular Degeneration by Inhibiting Retinal Pigment Epithelium Cell Death and Autophagy Alterations.Cells. 2020 Jul 4;9(7):1617. doi: 10.3390/cells9071617. Cells. 2020. PMID: 32635502 Free PMC article.
-
Size-Dependent Cytotoxicity and Reactive Oxygen Species of Cerium Oxide Nanoparticles in Human Retinal Pigment Epithelia Cells.Int J Nanomedicine. 2021 Aug 10;16:5333-5341. doi: 10.2147/IJN.S305676. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34408413 Free PMC article.
-
Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model.Exp Eye Res. 2021 Feb;203:108391. doi: 10.1016/j.exer.2020.108391. Epub 2020 Dec 8. Exp Eye Res. 2021. PMID: 33307075
-
The use of cerium oxide nanoparticles in liver disorders: A double-sided coin?Basic Clin Pharmacol Toxicol. 2022 Mar;130(3):349-363. doi: 10.1111/bcpt.13700. Epub 2022 Jan 13. Basic Clin Pharmacol Toxicol. 2022. PMID: 34902883 Review.
-
Utilization of Nanoparticles for Treating Age-Related Macular Degeneration.Pharmaceuticals (Basel). 2025 Jan 25;18(2):162. doi: 10.3390/ph18020162. Pharmaceuticals (Basel). 2025. PMID: 40005976 Free PMC article. Review.
Cited by
-
Emerging innovations in ophthalmic drug delivery for diabetic retinopathy: a translational perspective.Drug Deliv Transl Res. 2025 Jul 20. doi: 10.1007/s13346-025-01925-6. Online ahead of print. Drug Deliv Transl Res. 2025. PMID: 40685494
-
Management of cardiovascular disease by cerium oxide nanoparticles via alleviating oxidative stress and adipokine abnormalities.Sci Rep. 2025 Feb 17;15(1):5709. doi: 10.1038/s41598-025-85794-6. Sci Rep. 2025. PMID: 39962072 Free PMC article.
-
Ceria nanoparticles: biomedical applications and toxicity.J Zhejiang Univ Sci B. 2024 May 15;25(5):361-388. doi: 10.1631/jzus.B2300854. J Zhejiang Univ Sci B. 2024. PMID: 38725338 Free PMC article. Review.
-
Comparative Study of Hydroxytyrosol Acetate and Hydroxytyrosol in Activating Phase II Enzymes.Antioxidants (Basel). 2023 Oct 7;12(10):1834. doi: 10.3390/antiox12101834. Antioxidants (Basel). 2023. PMID: 37891913 Free PMC article.
-
Emerging Promise of Green Synthesized Metallic Nanoparticles for the Management of Neurological Disorders.Curr Pharm Des. 2025;31(5):344-359. doi: 10.2174/0113816128337464240930042205. Curr Pharm Des. 2025. PMID: 39400023 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

