Adipocyte-Specific Expression of PGC1α Promotes Adipocyte Browning and Alleviates Obesity-Induced Metabolic Dysfunction in an HO-1-Dependent Fashion
- PMID: 35740043
- PMCID: PMC9220759
- DOI: 10.3390/antiox11061147
Adipocyte-Specific Expression of PGC1α Promotes Adipocyte Browning and Alleviates Obesity-Induced Metabolic Dysfunction in an HO-1-Dependent Fashion
Abstract
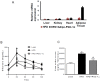
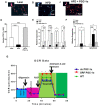
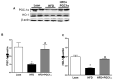
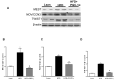
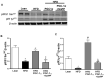
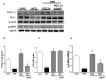
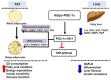
Recent studies suggest that PGC1-α plays a crucial role in mitochondrial and vascular function, yet the physiological significance of PGC1α and HO expression in adipose tissues in the context of obesity-linked vascular dysfunction remains unclear. We studied three groups of six-week-old C57BL/6J male mice: (1) mice fed a normal chow diet; (2) mice fed a high-fat diet (H.F.D.) for 28 weeks, and (3) mice fed a high-fat diet (H.F.D.) for 28 weeks, treated with adipose-specific overexpression of PGC-1α (transgenic-adipocyte-PGC-1α) at week 20, and continued on H.F.D. for weeks 20-28. R.N.A. arrays examined 88 genes involved in adipocyte proliferation and maturation. Blood pressure, tissue fibrosis, fasting glucose, and oxygen consumption were measured, as well as liver steatosis, and the expression levels of metabolic and mitochondrial markers. Obese mice exhibited a marked reduction of PGC1α and developed adipocyte hypertrophy, fibrosis, hepatic steatosis, and decreased mitochondrial respiration. Mice with adipose-specific overexpression of PGC1-α exhibited improvement in HO-1, mitochondrial biogenesis and respiration, with a decrease in fasting glucose, reduced blood pressure and fibrosis, and increased oxygen consumption. PGC-1α led to the upregulated expression of processes associated with the browning of fat tissue, including UCP1, FGF21, and pAMPK signaling, with a reduction in inflammatory adipokines, NOV/CCN3 expression, and TGFβ. These changes required HO-1 expression. The R.N.A. array analysis identified subgroups of genes positively correlated with contributions to the browning of adipose tissue, all dependent on HO-1. Our observations reveal a positive impact of adipose-PGC1-α on distal organ systems, with beneficial effects on HO-1 levels, reversing obesity-linked cardiometabolic disturbances.
Keywords: PGC-1α; brown fat; inflammation; mitochondria; obesity; type 2 diabetes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures












Similar articles
-
Silencing the Adipocytokine NOV: A Novel Approach to Reversing Oxidative Stress-Induced Cardiometabolic Dysfunction.Cells. 2022 Sep 29;11(19):3060. doi: 10.3390/cells11193060. Cells. 2022. PMID: 36231029 Free PMC article.
-
Ablation of adipose-HO-1 expression increases white fat over beige fat through inhibition of mitochondrial fusion and of PGC1α in female mice.Horm Mol Biol Clin Investig. 2017 Aug 1;31(1). doi: 10.1515/hmbci-2017-0027. Horm Mol Biol Clin Investig. 2017. PMID: 28763300
-
Cold Press Pomegranate Seed Oil Attenuates Dietary-Obesity Induced Hepatic Steatosis and Fibrosis through Antioxidant and Mitochondrial Pathways in Obese Mice.Int J Mol Sci. 2020 Jul 31;21(15):5469. doi: 10.3390/ijms21155469. Int J Mol Sci. 2020. PMID: 32751794 Free PMC article.
-
Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy.Front Endocrinol (Lausanne). 2015 Jan 30;6:1. doi: 10.3389/fendo.2015.00001. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25688231 Free PMC article. Review.
-
HDL Mimetics Enhances Mitochondrial Function via Stimulation of PGC1-α.Eur Cardiol. 2023 Apr 25;18:e27. doi: 10.15420/ecr.2023.18.PO10. eCollection 2023. Eur Cardiol. 2023. PMID: 37405353 Free PMC article. Review. No abstract available.
Cited by
-
The Other Side of the Perfect Cup: Coffee-Derived Non-Polyphenols and Their Roles in Mitigating Factors Affecting the Pathogenesis of Type 2 Diabetes.Int J Mol Sci. 2024 Aug 17;25(16):8966. doi: 10.3390/ijms25168966. Int J Mol Sci. 2024. PMID: 39201652 Free PMC article. Review.
-
Adipose tissue dysfunction disrupts metabolic homeostasis: mechanisms linking fat dysregulation to disease.Front Endocrinol (Lausanne). 2025 Jun 24;16:1592683. doi: 10.3389/fendo.2025.1592683. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40630101 Free PMC article. Review.
-
Jiangtang Sanhao formula ameliorates skeletal muscle insulin resistance via regulating GLUT4 translocation in diabetic mice.Front Pharmacol. 2022 Sep 8;13:950535. doi: 10.3389/fphar.2022.950535. eCollection 2022. Front Pharmacol. 2022. PMID: 36160420 Free PMC article.
-
The Role of Epicardial Adipose Tissue in Acute Coronary Syndromes, Post-Infarct Remodeling and Cardiac Regeneration.Int J Mol Sci. 2024 Mar 22;25(7):3583. doi: 10.3390/ijms25073583. Int J Mol Sci. 2024. PMID: 38612394 Free PMC article. Review.
-
Two Regions with Different Expression of Lipogenic Enzymes in Rats' Posterior Subcutaneous Fat Depot.Int J Mol Sci. 2024 Oct 27;25(21):11546. doi: 10.3390/ijms252111546. Int J Mol Sci. 2024. PMID: 39519099 Free PMC article.
References
-
- Ladhani M., Craig J.C., Irving M., Clayton P.A., Wong G. Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: A systematic review and meta-analysis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2017;32:439–449. doi: 10.1093/ndt/gfw075. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

