NADPH Oxidases in Pain Processing
- PMID: 35740059
- PMCID: PMC9219759
- DOI: 10.3390/antiox11061162
NADPH Oxidases in Pain Processing
Abstract
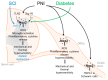
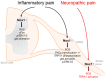
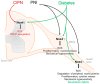
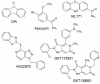
Inflammation or injury to the somatosensory nervous system may result in chronic pain conditions, which affect millions of people and often cause major health problems. Emerging lines of evidence indicate that reactive oxygen species (ROS), such as superoxide anion or hydrogen peroxide, are produced in the nociceptive system during chronic inflammatory and neuropathic pain and act as specific signaling molecules in pain processing. Among potential ROS sources in the somatosensory system are NADPH oxidases, a group of electron-transporting transmembrane enzymes whose sole function seems to be the generation of ROS. Interestingly, the expression and relevant function of the Nox family members Nox1, Nox2, and Nox4 in various cells of the nociceptive system have been demonstrated. Studies using knockout mice or specific knockdown of these isoforms indicate that Nox1, Nox2, and Nox4 specifically contribute to distinct signaling pathways in chronic inflammatory and/or neuropathic pain states. As selective Nox inhibitors are currently being developed and investigated in various physiological and pathophysiological settings, targeting Nox1, Nox2, and/or Nox4 could be a novel strategy for the treatment of chronic pain. Here, we summarize the distinct roles of Nox1, Nox2, and Nox4 in inflammatory and neuropathic processing and discuss the effectiveness of currently available Nox inhibitors in the treatment of chronic pain conditions.
Keywords: NADPH oxidase; Nox; Nox inhibition; inflammation; neuropathy; nociception; pain; peripheral injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Finnerup N.B., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R.H., Gilron I., Haanpää M., Hansson P., Jensen T.S., et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

