Measurement of Tetrahydrobiopterin in Animal Tissue Samples by HPLC with Electrochemical Detection-Protocol Optimization and Pitfalls
- PMID: 35740082
- PMCID: PMC9228106
- DOI: 10.3390/antiox11061182
Measurement of Tetrahydrobiopterin in Animal Tissue Samples by HPLC with Electrochemical Detection-Protocol Optimization and Pitfalls
Abstract
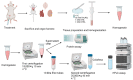
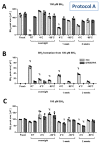
Tetrahydrobiopterin (BH4) is an essential cofactor of all nitric oxide synthase isoforms, thus determination of BH4 levels can provide important mechanistic insight into diseases. We established a protocol for high-performance liquid chromatography/electrochemical detection (HPLC/ECD)-based determination of BH4 in tissue samples. We first determined the optimal storage and work-up conditions for authentic BH4 and its oxidation product dihydrobiopterin (BH2) under various conditions (pH, temperature, presence of antioxidants, metal chelators, and storage time). We then applied optimized protocols for detection of BH4 in tissues of septic (induced by lipopolysaccharide [LPS]) rats. BH4 standards in HCl are stabilized by addition of 1,4-dithioerythritol (DTE) and diethylenetriaminepentaacetic acid (DTPA), while HCl was sufficient for BH2 standard stabilization. Overnight storage of BH4 standard solutions at room temperature in HCl without antioxidants caused complete loss of BH4 and the formation of BH2. We further optimized the protocol to separate ascorbate and the BH4 tissue sample and found a significant increase in BH4 in the heart and kidney as well as higher BH4 levels by trend in the brain of septic rats compared to control rats. These findings correspond to reports on augmented nitric oxide and BH4 levels in both animals and patients with septic shock.
Keywords: HPLC with electrochemical detection; oxidative stress; sepsis; tetrahydrobiopterin.
Conflict of interest statement
The authors declare that they have no conflict of interest with the contents of this article. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Tetrahydrobiopterin Supplementation: Elevation of Tissue Biopterin Levels Accompanied by a Relative Increase in Dihydrobiopterin in the Blood and the Role of Probenecid-Sensitive Uptake in Scavenging Dihydrobiopterin in the Liver and Kidney of Rats.PLoS One. 2016 Oct 6;11(10):e0164305. doi: 10.1371/journal.pone.0164305. eCollection 2016. PLoS One. 2016. PMID: 27711248 Free PMC article.
-
Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways.J Biol Chem. 2009 Oct 9;284(41):28128-28136. doi: 10.1074/jbc.M109.041483. Epub 2009 Aug 7. J Biol Chem. 2009. PMID: 19666465 Free PMC article.
-
Simplified HPLC methodology for quantifying biological pterins by selective oxidation.J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Jun 15;1055-1056:113-118. doi: 10.1016/j.jchromb.2017.04.018. Epub 2017 Apr 24. J Chromatogr B Analyt Technol Biomed Life Sci. 2017. PMID: 28460363
-
Delivery of exogenous tetrahydrobiopterin (BH4) to cells of target organs: role of salvage pathway and uptake of its precursor in effective elevation of tissue BH4.Mol Genet Metab. 2005 Dec;86 Suppl 1:S2-10. doi: 10.1016/j.ymgme.2005.09.002. Epub 2005 Oct 25. Mol Genet Metab. 2005. PMID: 16256391 Review.
-
[The biological effect of tetrahydrobiopterin and its potential role in sepsis].Sheng Li Ke Xue Jin Zhan. 1999 Oct;30(4):303-8. Sheng Li Ke Xue Jin Zhan. 1999. PMID: 12532822 Review. Chinese.
Cited by
-
Targeting the ferroptosis pathway for rheumatoid arthritis: molecular mechanisms and prospects for inhibitor development.Front Immunol. 2025 Jun 10;16:1610121. doi: 10.3389/fimmu.2025.1610121. eCollection 2025. Front Immunol. 2025. PMID: 40557156 Free PMC article. Review.
-
Insights into Molecular Structure of Pterins Suitable for Biomedical Applications.Int J Mol Sci. 2022 Dec 3;23(23):15222. doi: 10.3390/ijms232315222. Int J Mol Sci. 2022. PMID: 36499560 Free PMC article. Review.
References
-
- Daiber A., Oelze M., Daub S., Steven S., Schuff A., Kroller-Schon S., Hausding M., Wenzel P., Schulz E., Gori T., et al. Vascular redox signaling, redox switches in endothelial nitric oxide synthase and endothelial dysfunction. In: Laher I., editor. Systems Biology of Free Radicals and Antioxidants. Springer; Berlin/Heidelberg, Germany: 2014. pp. 1177–1211.
Grants and funding
LinkOut - more resources
Full Text Sources

