Antitumor Properties of a New Macrocyclic Tetranuclear Oxidovanadium(V) Complex with 3-Methoxysalicylidenvaline Ligand
- PMID: 35740239
- PMCID: PMC9220379
- DOI: 10.3390/biomedicines10061217
Antitumor Properties of a New Macrocyclic Tetranuclear Oxidovanadium(V) Complex with 3-Methoxysalicylidenvaline Ligand
Abstract
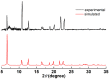
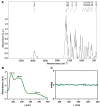
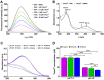
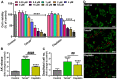
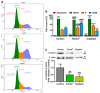
A wide variety of metal-based compounds have been obtained and studied for their antitumor activity since the intensely used cytostatic drugs (e.g., cisplatin) failed to accomplish their expected pharmacological properties. Thus, we aimed to develop a new vanadium-based drug and assess its antitumor properties using the human hepatocarcinoma (HepG2) cell line. The compound was synthesized from vanadyl sulfate, DL-valine, and o-vanillin and was spectrally and structurally characterized (UV-Vis, IR, CD, and single-crystal/powder-XRD). Compound stability in biological media, cell uptake, and the interaction with albumin were assessed. The mechanisms of its antitumor activity were determined compared to cisplatin by performing cytotoxicity, oxidative and mitochondrial status, DNA fragmentation, β-Tubulin synthesis investigation, and cell cycle studies. Herein, we developed a macrocyclic tetranuclear oxidovanadium(V) compound, [(VVO)(L)(CH3O)]4, having coordinated four Schiff base (H2L) ligands, 3-methoxysalicylidenvaline. We showed that [(VVO)(L)(CH3O)]4: (i) has pH-dependent stability in biological media, (ii) binds to albumin in a dose-dependent manner, (iii) is taken up by cells in a time-dependent way, (iv) has a higher capacity to induce cell death compared to cisplatin (IC50 = 6 μM vs. 10 μM), by altering the oxidative and mitochondrial status in HepG2 cells. Unlike cisplatin, which blocks the cell cycle in the S-phase, the new vanadium-based compound arrests it in S and G2/M-phase, whereas no differences in the induction of DNA fragmentation and reduction of β-Tubulin synthesis between the two were determined. Thus, the [(VVO)(L)(CH3O)]4 antitumor mechanism involved corroboration between the generation of oxidative species, mitochondrial dysfunction, degradation of DNA, cell cycle arrest in the S and G2/M-phase, and β-Tubulin synthesis reduction. Our studies demonstrate the potent antitumor activity of [(VVO)(L)(CH3O)]4 and propose it as an attractive candidate for anticancer therapy.
Keywords: 3-methoxysalicylidenvaline ligand; antitumor activity; apoptosis; hepatocarcinoma; oxidovanadium(V) complex.
Conflict of interest statement
The authors of this paper have nothing to disclose.
Figures




References
-
- Kudo M., Ueshima K., Yokosuka O., Ogasawara S., Obi S., Izumi N., Aikata H., Nagano H., Hatano E., Sasaki Y., et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018;3:424–432. doi: 10.1016/S2468-1253(18)30078-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

