Chemical Exchange Saturation Transfer (CEST) Signal at -1.6 ppm and Its Application for Imaging a C6 Glioma Model
- PMID: 35740241
- PMCID: PMC9219881
- DOI: 10.3390/biomedicines10061220
Chemical Exchange Saturation Transfer (CEST) Signal at -1.6 ppm and Its Application for Imaging a C6 Glioma Model
Abstract
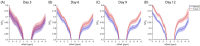
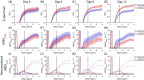
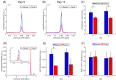
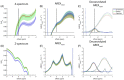
The chemical exchange saturation transfer (CEST) signal at -1.6 ppm is attributed to the choline methyl on phosphatidylcholines and results from the relayed nuclear Overhauser effect (rNOE), that is, rNOE(-1.6). The formation of rNOE(-1.6) involving the cholesterol hydroxyl is shown in liposome models. We aimed to confirm the correlation between cholesterol content and rNOE(-1.6) in cell cultures, tissues, and animals. C57BL/6 mice (N = 9) bearing the C6 glioma tumor were imaged in a 7 T MRI scanner, and their rNOE(-1.6) images were cross-validated through cholesterol staining with filipin. Cholesterol quantification was obtained using an 18.8-T NMR spectrometer from the lipid extracts of the brain tissues from another group of mice (N = 3). The cholesterol content in the cultured cells was manipulated using methyl-β-cyclodextrin and a complex of cholesterol and methyl-β-cyclodextrin. The rNOE(-1.6) of the cell homogenates and their cholesterol levels were measured using a 9.4-T NMR spectrometer. The rNOE(-1.6) signal is hypointense in the C6 tumors of mice, which matches the filipin staining results, suggesting that their tumor region is cholesterol deficient. The tissue extracts also indicate less cholesterol and phosphatidylcholine contents in tumors than in normal brain tissues. The amplitude of rNOE(-1.6) is positively correlated with the cholesterol concentration in the cholesterol-manipulated cell cultures. Our results indicate that the cholesterol dependence of rNOE(-1.6) occurs in cell cultures and solid tumors of C6 glioma. Furthermore, when the concentration of phosphatidylcholine is carefully considered, rNOE(-1.6) can be developed as a cholesterol-weighted imaging technique.
Keywords: chemical exchange saturation transfer (CEST); cholesterol; glioma; magnetic resonance imaging (MRI); relayed nuclear Overhauser effect (rNOE).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Role of the cholesterol hydroxyl group in the chemical exchange saturation transfer signal at -1.6 ppm.NMR Biomed. 2020 Sep;33(9):e4356. doi: 10.1002/nbm.4356. Epub 2020 Jun 23. NMR Biomed. 2020. PMID: 32575161
-
Role of chemical exchange on the relayed nuclear Overhauser enhancement signal in saturation transfer MRI.Magn Reson Med. 2022 Jan;87(1):365-376. doi: 10.1002/mrm.28961. Epub 2021 Aug 12. Magn Reson Med. 2022. PMID: 34382694 Free PMC article.
-
Accuracy in the quantification of chemical exchange saturation transfer (CEST) and relayed nuclear Overhauser enhancement (rNOE) saturation transfer effects.NMR Biomed. 2017 Jul;30(7):10.1002/nbm.3716. doi: 10.1002/nbm.3716. Epub 2017 Mar 8. NMR Biomed. 2017. PMID: 28272761 Free PMC article.
-
The relayed nuclear Overhauser effect in magnetization transfer and chemical exchange saturation transfer MRI.NMR Biomed. 2023 Jun;36(6):e4778. doi: 10.1002/nbm.4778. Epub 2022 Jun 20. NMR Biomed. 2023. PMID: 35642102 Free PMC article. Review.
-
Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum.Neuroimage. 2018 Mar;168:222-241. doi: 10.1016/j.neuroimage.2017.04.045. Epub 2017 Apr 21. Neuroimage. 2018. PMID: 28435103 Free PMC article. Review.
References
-
- Xu X., Chan K.W., Knutsson L., Artemov D., Xu J., Liu G., Kato Y., Lal B., Laterra J., McMahon M. Dynamic glucose enhanced (DGE) MRI for combined imaging of blood-brain barrier break down and increased blood volume in brain cancer. Magn. Reson. Med. 2015;74:1556–1563. doi: 10.1002/mrm.25995. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

