Preclinical Studies of Granulysin-Based Anti-MUC1-Tn Immunotoxins as a New Antitumoral Treatment
- PMID: 35740244
- PMCID: PMC9219680
- DOI: 10.3390/biomedicines10061223
Preclinical Studies of Granulysin-Based Anti-MUC1-Tn Immunotoxins as a New Antitumoral Treatment
Abstract
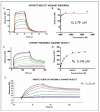
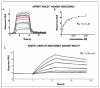
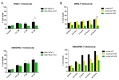
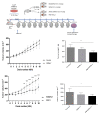
Two granulysin (GRNLY) based immunotoxins were generated, one containing the scFv of the SM3 mAb (SM3GRNLY) and the other the scFv of the AR20.5 mAb (AR20.5GRNLY). These mAb recognize different amino acid sequences of aberrantly O-glycosylated MUC1, also known as the Tn antigen, expressed in a variety of tumor cell types. We first demonstrated the affinity of these immunotoxins for their antigen using surface plasmon resonance for the purified antigen and flow cytometry for the antigen expressed on the surface of living tumor cells. The induction of cell death of tumor cell lines of different origin positive for Tn antigen expression was stronger in the cases of the immunotoxins than that induced by GRNLY alone. The mechanism of cell death induced by the immunotoxins was studied, showing that the apoptotic component demonstrated previously for GRNLY was also present, but that cell death induced by the immunotoxins included also necroptotic and necrotic components. Finally, we demonstrated the in vivo tumor targeting by the immunotoxins after systemic injection using a xenograft model of the human pancreatic adenocarcinoma CAPAN-2 in athymic mice. While GRNLY alone did not have a therapeutic effect, SM3GRNLY and AR20.5GRNLY reduced tumor volume by 42 and 60%, respectively, compared with untreated tumor-bearing mice, although the results were not statistically significant in the case of AR20.5GRNLY. Histological studies of tumors obtained from treated mice demonstrated reduced cellularity, nuclear morphology compatible with apoptosis induction and active caspase-3 detection by immunohistochemistry. Overall, our results exemplify that these immunotoxins are potential drugs to treat Tn-expressing cancers.
Keywords: MUC1; Tn antigen; granulysin; immunotoxins.
Conflict of interest statement
The use of granulysin immunotoxins as an anti-tumoral treatment is protected by the patent application PCT/ES2018/P201830768 presented to the Spanish Bureau of Patents and Brands (OEPM) on 26 July 2018; international application number PCT/EP2019/069979; number of publication WO 2020020978 A1.
Figures






References
-
- Martínez-Sáez N., Castro-López J., Valero-González J., Madariaga D., Compañón I., Somovilla V., Salvadó M., Asensio J., Jiménez-Barbero J., Avenoza A., et al. Deciphering the Non-Equivalence of Serine and Threonine O-Glycosylation Points: Implications for Molecular Recognition of the Tn Antigen by an anti-MUC1 Antibody. Angew. Chem. Int. Ed. Engl. 2015;54:9830–9834. doi: 10.1002/anie.201502813. - DOI - PMC - PubMed
-
- Cheever M., Allison J., Ferris A., Finn O., Hastings B., Hecht T., Mellman I., Prindiville S., Viner J., Weiner L., et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

