One Molecule for Mental Nourishment and More: Glucose Transporter Type 1-Biology and Deficiency Syndrome
- PMID: 35740271
- PMCID: PMC9219734
- DOI: 10.3390/biomedicines10061249
One Molecule for Mental Nourishment and More: Glucose Transporter Type 1-Biology and Deficiency Syndrome
Abstract
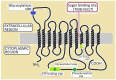
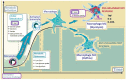
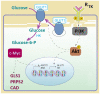
Glucose transporter type 1 (Glut1) is the main transporter involved in the cellular uptake of glucose into many tissues, and is highly expressed in the brain and in erythrocytes. Glut1 deficiency syndrome is caused mainly by mutations of the SLC2A1 gene, impairing passive glucose transport across the blood-brain barrier. All age groups, from infants to adults, may be affected, with age-specific symptoms. In its classic form, the syndrome presents as an early-onset drug-resistant metabolic epileptic encephalopathy with a complex movement disorder and developmental delay. In later-onset forms, complex motor disorder predominates, with dystonia, ataxia, chorea or spasticity, often triggered by fasting. Diagnosis is confirmed by hypoglycorrhachia (below 45 mg/dL) with normal blood glucose, 18F-fluorodeoxyglucose positron emission tomography, and genetic analysis showing pathogenic SLC2A1 variants. There are also ongoing positive studies on erythrocytes' Glut1 surface expression using flow cytometry. The standard treatment still consists of ketogenic therapies supplying ketones as alternative brain fuel. Anaplerotic substances may provide alternative energy sources. Understanding the complex interactions of Glut1 with other tissues, its signaling function for brain angiogenesis and gliosis, and the complex regulation of glucose transportation, including compensatory mechanisms in different tissues, will hopefully advance therapy. Ongoing research for future interventions is focusing on small molecules to restore Glut1, metabolic stimulation, and SLC2A1 transfer strategies. Newborn screening, early identification and treatment could minimize the neurodevelopmental disease consequences. Furthermore, understanding Glut1 relative deficiency or inhibition in inflammation, neurodegenerative disorders, and viral infections including COVID-19 and other settings could provide clues for future therapeutic approaches.
Keywords: Glut1; SLC2A1; cognitive impairment; epilepsy; flow cytometry; glucose uptake; inborn errors of metabolism; inflammation; ketogenic diet; movement disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Landowski C.P., Suzuki Y., Hediger M.A. The Mammalian Transporter Families. In: Alpern R.J., Hebert S.C., editors. Seldin and Giebisch’s The Kidney: Physiology & Pathophysiology. 4th ed. Elsevier; Oxford, UK: 2008. pp. 91–146.
-
- De Vivo D.C., Trifiletti R.R., Jacobson R.I., Ronen G.M., Behmand R.A., Harik S.I. Defective Glucose Transport across the Blood-Brain Barrier as a Cause of Persistent Hypoglycorrhachia, Seizures, and Developmental Delay. N. Engl. J. Med. 1991;325:703–709. doi: 10.1056/NEJM199109053251006. - DOI - PubMed
-
- Klepper J., Akman C., Armeno M., Auvin S., Cervenka M., Cross H.J., De Giorgis V., Della Marina A., Engelstad K., Heussinger N., et al. Glut1 Deficiency Syndrome (Glut1DS): State of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open. 2020;5:354–365. doi: 10.1002/epi4.12414. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

