Rifaximin Improves Spatial Learning and Memory Impairment in Rats with Liver Damage-Associated Neuroinflammation
- PMID: 35740285
- PMCID: PMC9219896
- DOI: 10.3390/biomedicines10061263
Rifaximin Improves Spatial Learning and Memory Impairment in Rats with Liver Damage-Associated Neuroinflammation
Abstract
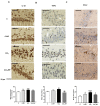
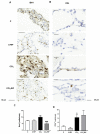
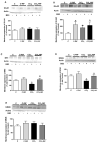
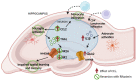
Patients with non-alcoholic fatty liver disease (NAFLD) may show mild cognitive impairment. Neuroinflammation in the hippocampus mediates cognitive impairment in rat models of minimal hepatic encephalopathy (MHE). Treatment with rifaximin reverses cognitive impairment in a large proportion of cirrhotic patients with MHE. However, the underlying mechanisms remain unclear. The aims of this work were to assess if rats with mild liver damage, as a model of NAFLD, show neuroinflammation in the hippocampus and impaired cognitive function, if treatment with rifaximin reverses it, and to study the underlying mechanisms. Mild liver damage was induced with carbon-tetrachloride. Infiltration of immune cells, glial activation, and cytokine expression, as well as glutamate receptors expression in the hippocampus and cognitive function were assessed. We assessed the effects of daily treatment with rifaximin on the alterations showed by these rats. Rats with mild liver damage showed hippocampal neuroinflammation, reduced membrane expression of glutamate N-methyl-D-aspartate (NMDA) receptor subunits, and impaired spatial memory. Increased C-C Motif Chemokine Ligand 2 (CCL2), infiltration of monocytes, microglia activation, and increased tumor necrosis factor α (TNFα) were reversed by rifaximin, that normalized NMDA receptor expression and improved spatial memory. Thus, rifaximin reduces neuroinflammation and improves cognitive function in rats with mild liver damage, being a promising therapy for patients with NAFLD showing mild cognitive impairment.
Keywords: NMDA receptor; hepatic encephalopathy; microglia; mild liver damage; rifaximin; spatial learning and memory.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Role of peripheral inflammation in minimal hepatic encephalopathy.Metab Brain Dis. 2024 Dec;39(8):1667-1677. doi: 10.1007/s11011-024-01417-5. Epub 2024 Aug 23. Metab Brain Dis. 2024. PMID: 39177864 Review.
-
Extracellular vesicles from mesenchymal stem cells improve neuroinflammation and neurotransmission in hippocampus and cognitive impairment in rats with mild liver damage and minimal hepatic encephalopathy.Stem Cell Res Ther. 2024 Dec 18;15(1):472. doi: 10.1186/s13287-024-04076-6. Stem Cell Res Ther. 2024. PMID: 39696620 Free PMC article.
-
Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: reversal by sulforaphane.J Neuroinflammation. 2016 Feb 16;13:41. doi: 10.1186/s12974-016-0505-y. J Neuroinflammation. 2016. PMID: 26883214 Free PMC article.
-
Rifaximin Prevents T-Lymphocytes and Macrophages Infiltration in Cerebellum and Restores Motor Incoordination in Rats with Mild Liver Damage.Biomedicines. 2021 Aug 12;9(8):1002. doi: 10.3390/biomedicines9081002. Biomedicines. 2021. PMID: 34440206 Free PMC article.
-
Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: Underlying mechanisms and therapeutic implications.Acta Physiol (Oxf). 2019 Jun;226(2):e13270. doi: 10.1111/apha.13270. Epub 2019 Mar 22. Acta Physiol (Oxf). 2019. PMID: 30830722 Review.
Cited by
-
Role of peripheral inflammation in minimal hepatic encephalopathy.Metab Brain Dis. 2024 Dec;39(8):1667-1677. doi: 10.1007/s11011-024-01417-5. Epub 2024 Aug 23. Metab Brain Dis. 2024. PMID: 39177864 Review.
-
A narrative review about cognitive impairment in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Another matter to face through a holistic approach.J Adv Res. 2025 Feb;68:231-240. doi: 10.1016/j.jare.2024.02.007. Epub 2024 Feb 17. J Adv Res. 2025. PMID: 38369241 Free PMC article. Review.
-
Cellular Pathogenesis of Hepatic Encephalopathy: An Update.Biomolecules. 2023 Feb 19;13(2):396. doi: 10.3390/biom13020396. Biomolecules. 2023. PMID: 36830765 Free PMC article. Review.
-
The effects of rifaximin and lactulose on the gut-liver-brain axis in rats with minimal hepatic encephalopathy.PLoS One. 2025 Jun 17;20(6):e0325988. doi: 10.1371/journal.pone.0325988. eCollection 2025. PLoS One. 2025. PMID: 40526631 Free PMC article.
-
Circular RNA Tmcc1 improves astrocytic glutamate metabolism and spatial memory via NF-κB and CREB signaling in a bile duct ligation mouse model: transcriptional and cellular analyses.J Neuroinflammation. 2023 May 22;20(1):121. doi: 10.1186/s12974-023-02806-w. J Neuroinflammation. 2023. PMID: 37217942 Free PMC article.
References
-
- Felipo V., Urios A., Montesinos E., Molina I., Garcia-Torres M.L., Civera M., Del Olmo J.A., Ortega J., Martinez-Valls J., Serra M.A., et al. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab. Brain Dis. 2012;27:51–58. doi: 10.1007/s11011-011-9269-3. - DOI - PubMed
-
- Giménez-Garzó C., Fiorillo A., Ballester-Ferré M.P., Gallego J.J., Casanova-Ferrer F., Urios A., Benlloch S., Martí-Aguado D., San-Miguel T., Tosca J., et al. A new score unveils a high prevalence of mild cognitive impairment in patients with nonalcoholic fatty liver disease. J. Clin. Med. 2021;10:2806. doi: 10.3390/jcm10132806. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

