Genetic Profiling of Glucocorticoid (NR3C1) and Mineralocorticoid (NR3C2) Receptor Polymorphisms before Starting Therapy with Androgen Receptor Inhibitors: A Study of a Patient Who Developed Toxic Myocarditis after Enzalutamide Treatment
- PMID: 35740293
- PMCID: PMC9220762
- DOI: 10.3390/biomedicines10061271
Genetic Profiling of Glucocorticoid (NR3C1) and Mineralocorticoid (NR3C2) Receptor Polymorphisms before Starting Therapy with Androgen Receptor Inhibitors: A Study of a Patient Who Developed Toxic Myocarditis after Enzalutamide Treatment
Abstract
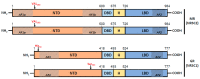
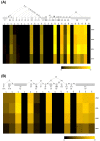
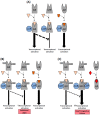
Enzalutamide is a nonsteroidal inhibitor of the androgen receptor (AR) signaling pathway and is used to treat patients with metastatic castration-resistant prostate cancer. However, the risk of cardiovascular-related hospitalization in patients with no contraindications for the use of enzalutamide is about 1-2%. To date, the underlying molecular basis of this has not been established. The androgen receptor, glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) are nuclear receptors that share structural similarities and have closely related DNA-binding sites and coregulators. In non-epithelial cells, a fine balance of the activities of these receptors is essential to ensure correct cellular function. In this study, we present a molecular characterization of these nuclear receptors in a prostate cancer patient who developed congestive heart failure after enzalutamide treatment. White cell RNAseq revealed a homozygous rs5522 MR polymorphism and both the rs143711342 and rs56149945 GR polymorphisms, carried in different alleles. No different specific splice isoforms were detected. Recent research suggests that AR inhibition by enzalutamide makes available a coregulator that specifically interacts with the rs5522-mutated MR, increasing its activity and producing adverse effects on cardiovascular health. We suggest an evaluation of the MR rs5522 polymorphism before starting therapy with AR inhibitors.
Keywords: aldosterone; androgen receptor; androgen receptor inhibitors; enzalutamide; glucocorticoid receptor; mineralocorticoid receptor; prostate cancer; spironolactone; toxic myocarditis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Scher H.I., Beer T.M., Higano C.S., Anand A., Taplin M.E., Efstathiou E., Rathkopf D., Shelkey J., Evan Y.Y., Alumkal J., et al. Antitumour activity of MDV3100 in castra-tion-resistant prostate cancer: A phase 1–2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. - DOI - PMC - PubMed
-
- Scailteux L.M., Despas F., Balusson F., Campillo-Gimenez B., Mathieu R., Vincendeau S., Happe A., Nowak E., Kerbrat S., Oger E. Hospitalization for adverse events under abiraterone or enzalutamide exposure in real-world setting: A French population-based study on prostate cancer patients. Br. J. Clin. Pharmacol. 2022;88:336–346. doi: 10.1111/bcp.14972. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

