Hepatic Mitochondrial Dysfunction and Risk of Liver Disease in an Ovine Model of "PCOS Males"
- PMID: 35740312
- PMCID: PMC9220073
- DOI: 10.3390/biomedicines10061291
Hepatic Mitochondrial Dysfunction and Risk of Liver Disease in an Ovine Model of "PCOS Males"
Abstract
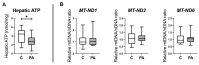
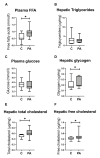
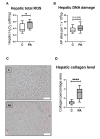
First-degree male relatives of polycystic ovary syndrome (PCOS) sufferers can develop metabolic abnormalities evidenced by elevated circulating cholesterol and triglycerides, suggestive of a male PCOS equivalent. Similarly, male sheep overexposed to excess androgens in fetal life develop dyslipidaemia in adolescence. Dyslipidaemia, altered lipid metabolism, and dysfunctional hepatic mitochondria are associated with the development of non-alcoholic liver disease (NAFLD). We therefore dissected hepatic mitochondrial function and lipid metabolism in adolescent prenatally androgenized (PA) males from an ovine model of PCOS. Testosterone was directly administered to male ovine fetuses to create prenatal androgenic overexposure. Liver RNA sequencing and proteomics occurred at 6 months of age. Hepatic lipids, glycogen, ATP, reactive oxygen species (ROS), DNA damage, and collagen were assessed. Adolescent PA males had an increased accumulation of hepatic cholesterol and glycogen, together with perturbed glucose and fatty acid metabolism, mitochondrial dysfunction, with altered mitochondrial transport, decreased oxidative phosphorylation and ATP synthesis, and impaired mitophagy. Mitochondrial dysfunction in PA males was associated with increased hepatic ROS level and signs of early liver fibrosis, with clinical relevance to NAFLD progression. We conclude that excess in utero androgen exposure in male fetuses leads to a PCOS-like metabolic phenotype with dysregulated mitochondrial function and likely lifelong health sequelae.
Keywords: NAFLD; NASH; androgens; hepatic cholesterol; liver fibrosis; male PCOS; mitochondrial dysfunction; oxidative phosphorylation; prenatal programming.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Pubertal FGF21 deficit is central in the metabolic pathophysiology of an ovine model of polycystic ovary syndrome.Mol Cell Endocrinol. 2021 Apr 5;525:111196. doi: 10.1016/j.mce.2021.111196. Epub 2021 Feb 6. Mol Cell Endocrinol. 2021. PMID: 33556473
-
Fetal androgen exposure is a determinant of adult male metabolic health.Sci Rep. 2019 Dec 27;9(1):20195. doi: 10.1038/s41598-019-56790-4. Sci Rep. 2019. PMID: 31882954 Free PMC article.
-
Mitochondrial gene polymorphisms alter hepatic cellular energy metabolism and aggravate diet-induced non-alcoholic steatohepatitis.Mol Metab. 2016 Feb 2;5(4):283-295. doi: 10.1016/j.molmet.2016.01.010. eCollection 2016 Apr. Mol Metab. 2016. PMID: 27069868 Free PMC article.
-
Mitochondrial dysfunction: An emerging link in the pathophysiology of polycystic ovary syndrome.Mitochondrion. 2020 May;52:24-39. doi: 10.1016/j.mito.2020.02.006. Epub 2020 Feb 17. Mitochondrion. 2020. PMID: 32081727 Review.
-
Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome?Hum Reprod Update. 2005 Jul-Aug;11(4):357-74. doi: 10.1093/humupd/dmi013. Hum Reprod Update. 2005. PMID: 15941725 Review.
Cited by
-
PCOS - the many faces of a disorder in women and men.J Endocrinol Invest. 2025 Apr;48(4):785-798. doi: 10.1007/s40618-024-02512-1. Epub 2024 Dec 16. J Endocrinol Invest. 2025. PMID: 39680364 Review.
-
Reactive oxygen species in polycystic ovary syndrome: Mechanistic insights into pathogenesis and therapeutic opportunities.Redox Biol. 2025 Sep;85:103776. doi: 10.1016/j.redox.2025.103776. Epub 2025 Jul 17. Redox Biol. 2025. PMID: 40694958 Free PMC article. Review.
-
Molecular Research on Polycystic Ovary Syndrome (PCOS).Biomedicines. 2023 May 4;11(5):1358. doi: 10.3390/biomedicines11051358. Biomedicines. 2023. PMID: 37239028 Free PMC article.
-
Genes in loci genetically associated with polycystic ovary syndrome are dynamically expressed in human fetal gonadal, metabolic and brain tissues.Front Endocrinol (Lausanne). 2023 May 8;14:1149473. doi: 10.3389/fendo.2023.1149473. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37223019 Free PMC article.
-
A meta-analysis: Effect of androgens on reproduction in sows.Front Endocrinol (Lausanne). 2023 Feb 10;14:1094466. doi: 10.3389/fendo.2023.1094466. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36843577 Free PMC article.
References
-
- Fauser B.C.J.M., Tarlatzis B.C., Rebar R.W., Legro R.S., Balen A.H., Lobo R., Carmina E., Chang J., Yildiz B.O., Laven J.S.E., et al. Consensus on Women’s Health Aspects of Polycystic Ovary Syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2011;97:28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024. - DOI - PubMed
-
- Crisosto N., Sir-Petermann T. Family Ties: Offspring Born to Women with Polycystic Ovary Syndrome. Curr. Opin. Endocr. Metab. Res. 2020;12:119–124. doi: 10.1016/j.coemr.2020.05.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

