Neurodynamic Treatment Promotes Mechanical Pain Modulation in Sensory Neurons and Nerve Regeneration in Rats
- PMID: 35740318
- PMCID: PMC9220043
- DOI: 10.3390/biomedicines10061296
Neurodynamic Treatment Promotes Mechanical Pain Modulation in Sensory Neurons and Nerve Regeneration in Rats
Abstract
Background: Somatic nerve injuries are a rising problem leading to disability associated with neuropathic pain commonly reported as mechanical allodynia (MA) and hyperalgesia. These symptoms are strongly dependent on specific processes in the dorsal root ganglia (DRG). Neurodynamic treatment (NDT), consisting of selective uniaxial nerve repeated tension protocols, effectively reduces pain and disability in neuropathic pain patients even though the biological mechanisms remain poorly characterized. We aimed to define, both in vivo and ex vivo, how NDT could promote nerve regeneration and modulate some processes in the DRG linked to MA and hyperalgesia.
Methods: We examined in Wistar rats, after unilateral median and ulnar nerve crush, the therapeutic effects of NDT and the possible protective effects of NDT administered for 10 days before the injury. We adopted an ex vivo model of DRG organotypic explant subjected to NDT to explore the selective effects on DRG cells.
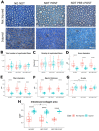
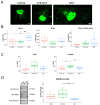
Results: Behavioural tests, morphological and morphometrical analyses, and gene and protein expression analyses were performed, and these tests revealed that NDT promotes nerve regeneration processes, speeds up sensory motor recovery, and modulates mechanical pain by affecting, in the DRG, the expression of TACAN, a mechanosensitive receptor shared between humans and rats responsible for MA and hyperalgesia. The ex vivo experiments have shown that NDT increases neurite regrowth and confirmed the modulation of TACAN.
Conclusions: The results obtained in this study on the biological and molecular mechanisms induced by NDT will allow the exploration, in future clinical trials, of its efficacy in different conditions of neuropathic pain.
Keywords: allodynia; dorsal root ganglia; gene expression; motor recovery; neurodynamic; neuropathic pain; nociceptor; non-pharmacological treatment; regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The neurodynamic treatment induces biological changes in sensory and motor neurons in vitro.Sci Rep. 2021 Jun 24;11(1):13277. doi: 10.1038/s41598-021-92682-2. Sci Rep. 2021. PMID: 34168249 Free PMC article.
-
Increased Expression of Fibronectin Leucine-Rich Transmembrane Protein 3 in the Dorsal Root Ganglion Induces Neuropathic Pain in Rats.J Neurosci. 2019 Sep 18;39(38):7615-7627. doi: 10.1523/JNEUROSCI.0295-19.2019. Epub 2019 Jul 25. J Neurosci. 2019. PMID: 31346030 Free PMC article.
-
Comparison of neuropathic pain and neuronal apoptosis following nerve root or spinal nerve compression.Eur Spine J. 2009 Dec;18(12):1978-85. doi: 10.1007/s00586-009-1064-z. Epub 2009 Jun 19. Eur Spine J. 2009. PMID: 19543754 Free PMC article.
-
Dorsal root ganglion application of muscimol prevents hyperalgesia and stimulates myelin protein expression after sciatic nerve injury in rats.Anesth Analg. 2012 Mar;114(3):674-82. doi: 10.1213/ANE.0b013e31823fad7e. Epub 2011 Dec 20. Anesth Analg. 2012. PMID: 22190549
-
Role of the immune system in neuropathic pain.Scand J Pain. 2019 Dec 18;20(1):33-37. doi: 10.1515/sjpain-2019-0138. Scand J Pain. 2019. PMID: 31730538 Review.
Cited by
-
Mechanisms of neurodynamic treatments (MONET): a protocol for a mechanistic, randomised, single-blind controlled trial in patients with carpal tunnel syndrome.BMC Musculoskelet Disord. 2024 Jul 27;25(1):590. doi: 10.1186/s12891-024-07713-6. BMC Musculoskelet Disord. 2024. PMID: 39068435 Free PMC article.
-
Neuromodulation Through Magnetic Fields Irradiation with AT-04 Improves Hyperalgesia in a Rat Model of Neuropathic Pain via Descending Pain Modulatory Systems and Opioid Analgesia.Cell Mol Neurobiol. 2023 Nov;43(8):4345-4362. doi: 10.1007/s10571-023-01430-9. Epub 2023 Nov 7. Cell Mol Neurobiol. 2023. PMID: 37934363 Free PMC article.
-
Mechanism, application and effect evaluation of nerve mobilization in the treatment of low back pain: A narrative review.Medicine (Baltimore). 2023 Aug 25;102(34):e34961. doi: 10.1097/MD.0000000000034961. Medicine (Baltimore). 2023. PMID: 37653794 Free PMC article. Review.
-
Perineural Electrical Dry Needling and Neural Mobilization for Chemotherapy-Induced Peripheral Neuropathy: Case Report.J Clin Med. 2025 Mar 28;14(7):2318. doi: 10.3390/jcm14072318. J Clin Med. 2025. PMID: 40217767 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

