Inhibiting Transglutaminase 2 Mediates Kidney Fibrosis via Anti-Apoptosis
- PMID: 35740367
- PMCID: PMC9220123
- DOI: 10.3390/biomedicines10061345
Inhibiting Transglutaminase 2 Mediates Kidney Fibrosis via Anti-Apoptosis
Abstract
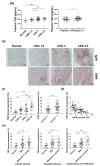
Transglutaminase 2 (TG2) is a calcium-dependent transamidating acyltransferase enzyme of the protein-glutamine γ-glutamyltransferase family implicated in kidney injury. In this study, we identified associations between TG2 and chronic kidney disease (CKD) identified by visualizing TG2 in kidney biopsy samples derived from CKD patients using immunohistochemistry and measuring the plasma TG2 concentrations. Our study revealed a connection between TG2 and the pathological markers of kidney disease. We showed high plasma TG2 levels in samples from patients with advanced CKD. In addition, we observed an increase in TG2 expression in tissues concomitant with advanced CKD in human samples. Moreover, we investigated the effect of TG2 inhibition on kidney injury using cystamine, a well-known competitive inhibitor of TG2. TG2 inhibition reduced apoptosis and accumulation of extracellular molecules (ECM) such as fibronectin and pro-inflammatory cytokine IL-8. Collectively, the increased expression of TG2 that was observed in advanced CKD, hence inhibiting TG2 activity, could protect kidney cells from ECM molecule accumulation, apoptosis, and inflammatory responses, thereby preventing kidney fibrosis.
Keywords: apoptosis; chronic kidney disease; cystamine; fibrosis; transglutaminase 2.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

