Isoliquiritigenin Inhibits Gastric Cancer Stemness, Modulates Tumor Microenvironment, and Suppresses Tumor Growth through Glucose-Regulated Protein 78 Downregulation
- PMID: 35740372
- PMCID: PMC9220208
- DOI: 10.3390/biomedicines10061350
Isoliquiritigenin Inhibits Gastric Cancer Stemness, Modulates Tumor Microenvironment, and Suppresses Tumor Growth through Glucose-Regulated Protein 78 Downregulation
Abstract
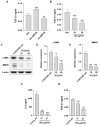
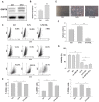
Chemotherapy is the treatment of choice for gastric cancer; however, the currently available therapeutic drugs for treatment have limited efficacy. Cancer stemness and the tumor microenvironment may play crucial roles in tumor growth and chemoresistance. Glucose-regulated protein 78 (GRP78) is an endoplasmic reticulum chaperone facilitating protein folding and cell homeostasis during stress and may participate in chemoresistance. Isoliquiritigenin (ISL) is a bioactive flavonoid found in licorice. In this study, we demonstrated the role of GRP78 in gastric cancer stemness and evaluated GRP78-mediated stemness inhibition, tumor microenvironment regulation, and chemosensitivity promotion by ISL. ISL not only suppressed GRP78-mediated gastric cancer stem cell-like characteristics, stemness-related protein expression, and cancer-associated fibroblast activation but also gastric tumor growth in xenograft animal studies. The findings indicated that ISL is a promising candidate for clinical use in combination chemotherapy.
Keywords: GRP78; cancer stemness; chemosensitivity; gastric cancer; isoliquiritigenin; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

