Chronic Rhinosinusitis, S. aureus Biofilm and Secreted Products, Inflammatory Responses, and Disease Severity
- PMID: 35740385
- PMCID: PMC9220248
- DOI: 10.3390/biomedicines10061362
Chronic Rhinosinusitis, S. aureus Biofilm and Secreted Products, Inflammatory Responses, and Disease Severity
Abstract
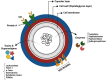
Chronic rhinosinusitis (CRS) is a persistent inflammation of the nasal cavity and paranasal sinuses associated with tissue remodelling, dysfunction of the sinuses' natural defence mechanisms, and induction of different inflammatory clusters. The etiopathogenesis of CRS remains elusive, and both environmental factors, such as bacterial biofilms and the host's general condition, are thought to play a role. Bacterial biofilms have significant clinical relevance due to their potential to cause resistance to antimicrobial therapy and host defenses. Despite substantial medical advances, some CRS patients suffer from recalcitrant disease that is unresponsive to medical and surgical treatments. Those patients often have nasal polyps with tissue eosinophilia, S. aureus-dominant mucosal biofilm, comorbid asthma, and a severely compromised quality of life. This review aims to summarise the contemporary knowledge of inflammatory cells/pathways in CRS, the role of bacterial biofilm, and their impact on the severity of the disease. Here, an emphasis is placed on S. aureus biofilm and its secreted products. A better understanding of these factors might offer important diagnostic and therapeutic perceptions for recalcitrant disease.
Keywords: S. aureus biofilm/virulence factor; chronic rhinosinusitis; inflammatory cells/endotypes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Staphylococcus aureus biofilm properties and chronic rhinosinusitis severity scores correlate positively with total CD4+ T-cell frequencies and inversely with its Th1, Th17 and regulatory cell frequencies.Immunology. 2023 Sep;170(1):120-133. doi: 10.1111/imm.13655. Epub 2023 May 16. Immunology. 2023. PMID: 37191458
-
S. aureus biofilm metabolic activity correlates positively with patients' eosinophil frequencies and disease severity in chronic rhinosinusitis.Microbes Infect. 2023 Nov-Dec;25(8):105213. doi: 10.1016/j.micinf.2023.105213. Epub 2023 Aug 29. Microbes Infect. 2023. PMID: 37652259
-
S. aureus biofilm properties correlate with immune B cell subset frequencies and severity of chronic rhinosinusitis.Clin Immunol. 2024 Jun;263:110221. doi: 10.1016/j.clim.2024.110221. Epub 2024 Apr 16. Clin Immunol. 2024. PMID: 38636891
-
Remodeling of Paranasal Sinuses Mucosa Functions in Response to Biofilm-Induced Inflammation.J Inflamm Res. 2024 Feb 26;17:1295-1323. doi: 10.2147/JIR.S443420. eCollection 2024. J Inflamm Res. 2024. PMID: 38434581 Free PMC article. Review.
-
Sinonasal Tissue Remodelling during Chronic Rhinosinusitis.Int J Otolaryngol. 2021 Sep 16;2021:7428955. doi: 10.1155/2021/7428955. eCollection 2021. Int J Otolaryngol. 2021. PMID: 34567126 Free PMC article. Review.
Cited by
-
Sustained co-release of ciprofloxacin and dexamethasone in rabbit maxillary sinus using polyvinyl alcohol-based hydrogel microparticle.J Mater Sci Mater Med. 2024 Sep 30;35(1):60. doi: 10.1007/s10856-024-06832-9. J Mater Sci Mater Med. 2024. PMID: 39348071 Free PMC article.
-
Hops bitter β-acids have antibacterial effects against sinonasal Staphylococcus aureus but also induce sinonasal cilia and mitochondrial dysfunction.Int Forum Allergy Rhinol. 2025 Mar;15(3):287-302. doi: 10.1002/alr.23487. Epub 2024 Nov 13. Int Forum Allergy Rhinol. 2025. PMID: 39533961 Free PMC article.
-
Periodontitis of maxillary teeth screened by community periodontal index is associated with chronic rhinosinusitis defined by EPOS 2020 guideline.Sci Rep. 2023 Oct 18;13(1):17722. doi: 10.1038/s41598-023-43474-3. Sci Rep. 2023. PMID: 37853005 Free PMC article.
-
Pathogenesis, Diagnosis, and Treatment of Infectious Rhinosinusitis.Microorganisms. 2024 Aug 16;12(8):1690. doi: 10.3390/microorganisms12081690. Microorganisms. 2024. PMID: 39203531 Free PMC article. Review.
-
[The correlation between preoperative neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio and postoperative recurrence of chronic rhinosinusitis].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024 Feb;38(2):133-139. doi: 10.13201/j.issn.2096-7993.2024.02.010. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024. PMID: 38297867 Free PMC article. Chinese.
References
-
- Rosenfeld R.M., Piccirillo J.F., Chandrasekhar S.S., Brook I., Ashok Kumar K., Kramper M., Orlandi R.R., Palmer J.N., Patel Z.M., Peters A. Clinical practice guideline (update) adult sinusitis executive summary. Otolaryngol.-Head Neck Surg. 2015;152:598–609. doi: 10.1177/0194599815574247. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

