Dietary Collagen Hydrolysates Retard Estrogen Deficiency-Induced Bone Loss through Blocking Osteoclastic Activation and Enhancing Osteoblastic Matrix Mineralization
- PMID: 35740404
- PMCID: PMC9219917
- DOI: 10.3390/biomedicines10061382
Dietary Collagen Hydrolysates Retard Estrogen Deficiency-Induced Bone Loss through Blocking Osteoclastic Activation and Enhancing Osteoblastic Matrix Mineralization
Abstract
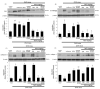
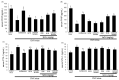
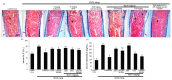
Osteoporosis manifest in postmenopausal women is an osteolytic disease characterized by bone loss, leading to increased susceptibility to bone fractures and frailty. The use of complementary therapies to alleviate postmenopausal osteoporosis is fairly widespread among women. The current study examined that Pangasius hypophthalmus fish skin collagen hydrolysates (fsCH) inhibited ovariectomy (OVX)-induced bone loss by conducting inter-comparative experiments for anti-osteoporotic activity among 206-618 mg/kg fsCH, 2 mg/kg isoflavone, 15 mg/kg glycine-proline-hydroxyproline (GPH) tripeptide, and calcium lactate. Surgical estrogen loss of mice for 8 weeks reduced serum 17β-estradiol levels with uterus atrophy, which was ameliorated by orally administering fsCH or isoflavone to mice. Similar to isoflavone, fsCH containing GPH-enhanced bone mineral density reduced levels of cathepsin K and proton-handling proteins, and elevated collagen 1 level in OVX bones. The treatment with fsCH and isoflavone enhanced the serum levels of collagen synthesis-related procollagen type 1 carboxy/amino-terminal propeptides reduced by OVX, whereas serum levels of osteocalcin and alkaline phosphatase, as well as collagen breakdown-related carboxy/amino-terminal telopeptides of type 1 collagen were reduced in OVX mice treated with fsCH, isoflavone, and calcium lactate. The trabecular bones were newly formed in OVX bones treated with isoflavone and fsCH, but not with calcium lactate. However, a low-dose combination of fsCH and calcium lactate had a beneficial synergy effect on postmenopausal osteoporosis. Furthermore, similar to isoflavone, 15-70 μg/mL fsCH, with its constituents of GPH and dipeptides of glycine-proline and proline-hydroxyproline, enhanced osteogenesis through stimulating differentiation, matrix mineralization, and calcium deposition of MC3T3-E1 osteoblasts. Accordingly, the presence of fsCH may encumber estrogen deficiency-induced bone loss through enhancing osteoclastogenic differentiation and matrix collagen synthesis. Therefore, fsCH may be a natural compound retarding postmenopausal osteoporosis and pathological osteoresorptive disorders.
Keywords: Pangasius hypophthalmus fish skin collagen hydrolysate; collagen; glycine–proline–hydroxyproline tripeptide; osteoblasts; ovariectomy; postmenopausal osteoporosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Cirsium Setidens Water Extracts Containing Linarin Block Estrogen Deprivation-Induced Bone Loss in Mice.Int J Mol Sci. 2023 Jan 13;24(2):1620. doi: 10.3390/ijms24021620. Int J Mol Sci. 2023. PMID: 36675135 Free PMC article.
-
Dietary Collagen Hydrolysates Ameliorate Furrowed and Parched Skin Caused by Photoaging in Hairless Mice.Int J Mol Sci. 2021 Jun 7;22(11):6137. doi: 10.3390/ijms22116137. Int J Mol Sci. 2021. PMID: 34200222 Free PMC article.
-
Estrogen enhances the bone regeneration potential of periodontal ligament stem cells derived from osteoporotic rats and seeded on nano-hydroxyapatite/collagen/poly(L-lactide).Int J Mol Med. 2016 Jun;37(6):1475-86. doi: 10.3892/ijmm.2016.2559. Epub 2016 Apr 12. Int J Mol Med. 2016. PMID: 27082697 Free PMC article.
-
Hydrolyzed collagen: Exploring its applications in the food and beverage industries and assessing its impact on human health - A comprehensive review.Heliyon. 2024 Aug 16;10(16):e36433. doi: 10.1016/j.heliyon.2024.e36433. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39253251 Free PMC article. Review.
-
A view on the skin-bone axis: unraveling similarities and potential of crosstalk.Front Med (Lausanne). 2024 Mar 4;11:1360483. doi: 10.3389/fmed.2024.1360483. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38500951 Free PMC article. Review.
Cited by
-
Review: Application of Protein-Based Raw Materials in Health Foods in China.Foods. 2024 Dec 25;14(1):20. doi: 10.3390/foods14010020. Foods. 2024. PMID: 39796310 Free PMC article. Review.
-
Eggshell membrane as promising supplement to maintain bone health: A systematic review.Bone Rep. 2024 May 24;21:101776. doi: 10.1016/j.bonr.2024.101776. eCollection 2024 Jun. Bone Rep. 2024. PMID: 38872992 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

