Macrophages Characterization in an Injured Bone Tissue
- PMID: 35740407
- PMCID: PMC9219779
- DOI: 10.3390/biomedicines10061385
Macrophages Characterization in an Injured Bone Tissue
Abstract
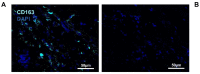
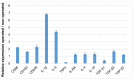
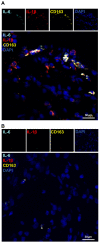
Biomaterial use is a promising approach to facilitate wound healing of the bone tissue. Biomaterials induce the formation of membrane capsules and the recruitment of different types of macrophages. Macrophages are immune cells that produce diverse combinations of cytokines playing an important role in bone healing and regeneration, but the exact mechanism remains to be studied. Our work aimed to identify in vivo macrophages in the Masquelet induced membrane in a rat model. Most of the macrophages in the damaged area were M2-like, with smaller numbers of M1-like macrophages. In addition, high expression of IL-1β and IL-6 cytokines were detected in the membrane region by RT-qPCR. Using an innovative combination of two hybridization techniques (in situ hybridization and in situ hybridization chain reaction (in situ HCR)), M2b-like macrophages were identified for the first time in cryosections of non-decalcified bone. Our work has also demonstrated that microspectroscopical analysis is essential for macrophage characterization, as it allows the discrimination of fluorescence and autofluorescence. Finally, this work has revealed the limitations of immunolabelling and the potential of in situ HCR to provide valuable information for in vivo characterization of macrophages.
Keywords: Masquelet induced membrane; bone; cryosection; cytokines; hybridization chain reaction (HCR); macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources

