Hippocampal Estrogen Signaling Mediates Sex Differences in Retroactive Interference
- PMID: 35740410
- PMCID: PMC9219958
- DOI: 10.3390/biomedicines10061387
Hippocampal Estrogen Signaling Mediates Sex Differences in Retroactive Interference
Abstract
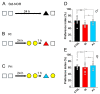
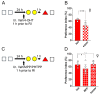
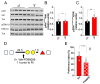
Despite being a crucial physiological function of the brain, the mechanisms underlying forgetting are still poorly understood. Estrogens play a critical role in different brain functions, including memory. However, the effects of sex hormones on forgetting vulnerabilitymediated by retroactive interference (RI), a phenomenon in which newly acquired information interferes with the retrieval of already stored information, are still poorly understood. The aim of our study was to characterize the sex differences in interference-mediated forgetting and identify the underlying molecular mechanisms. We found that adult male C57bl/6 mice showed a higher susceptibility to RI-dependent memory loss than females. The preference index (PI) in the NOR paradigm was 52.7 ± 5.9% in males and 62.3 ± 13.0% in females. The resistance to RI in female mice was mediated by estrogen signaling involving estrogen receptor α activation in the dorsal hippocampus. Accordingly, following RI, females showed higher phosphorylation levels (+30%) of extracellular signal-regulated kinase1/2 (ERK1/2) in the hippocampus. Pharmacological inhibition of ERK1/2 made female mice prone to RI. The PI was 70.6 ± 11.0% in vehicle-injected mice and 47.4 ± 10.8% following PD98059 administration. Collectively, our data suggest that hippocampal estrogen α receptor-ERK1/2 signaling is critically involved in a pattern separation mechanism that inhibits object-related RI in female mice.
Keywords: ERK1/2; estrogens; forgetting; hippocampus; object recognition memory; retroactive interference; sex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Memory-Related Synaptic Plasticity Is Sexually Dimorphic in Rodent Hippocampus.J Neurosci. 2018 Sep 12;38(37):7935-7951. doi: 10.1523/JNEUROSCI.0801-18.2018. Epub 2018 Aug 1. J Neurosci. 2018. PMID: 30209204 Free PMC article.
-
Memory Susceptibility to Retroactive Interference Is Developmentally Regulated by NMDA Receptors.Cell Rep. 2019 Feb 19;26(8):2052-2063.e4. doi: 10.1016/j.celrep.2019.01.098. Cell Rep. 2019. PMID: 30784588
-
17β-Estradiol and Agonism of G-protein-Coupled Estrogen Receptor Enhance Hippocampal Memory via Different Cell-Signaling Mechanisms.J Neurosci. 2016 Mar 16;36(11):3309-21. doi: 10.1523/JNEUROSCI.0257-15.2016. J Neurosci. 2016. PMID: 26985039 Free PMC article.
-
Regulation of object recognition and object placement by ovarian sex steroid hormones.Behav Brain Res. 2015 May 15;285:140-57. doi: 10.1016/j.bbr.2014.08.001. Epub 2014 Aug 15. Behav Brain Res. 2015. PMID: 25131507 Free PMC article. Review.
-
Neural, Cellular and Molecular Mechanisms of Active Forgetting.Front Syst Neurosci. 2018 Feb 6;12:3. doi: 10.3389/fnsys.2018.00003. eCollection 2018. Front Syst Neurosci. 2018. PMID: 29467630 Free PMC article. Review.
Cited by
-
Engineering memory with an extrinsically disordered kinase.Sci Adv. 2023 Nov 17;9(46):eadh1110. doi: 10.1126/sciadv.adh1110. Epub 2023 Nov 15. Sci Adv. 2023. PMID: 37967196 Free PMC article.
-
Acute stress alters recognition memory and AMPA/NMDA receptor subunits in a sex-dependent manner.Neurobiol Stress. 2023 May 26;25:100545. doi: 10.1016/j.ynstr.2023.100545. eCollection 2023 Jul. Neurobiol Stress. 2023. PMID: 37293561 Free PMC article.
-
High fat diet affects the hippocampal expression of miRNAs targeting brain plasticity-related genes.Sci Rep. 2024 Aug 23;14(1):19651. doi: 10.1038/s41598-024-69707-7. Sci Rep. 2024. PMID: 39179650 Free PMC article.
-
Paternal preconceptional diet enriched with n-3 polyunsaturated fatty acids affects offspring brain function in mice.Front Nutr. 2022 Oct 28;9:969848. doi: 10.3389/fnut.2022.969848. eCollection 2022. Front Nutr. 2022. PMID: 36386900 Free PMC article.
-
Maternal High Fat Diet Anticipates the AD-like Phenotype in 3xTg-AD Mice by Epigenetic Dysregulation of Aβ Metabolism.Cells. 2023 Jan 4;12(2):220. doi: 10.3390/cells12020220. Cells. 2023. PMID: 36672155 Free PMC article.
References
-
- Anderson M.C., Neely J.H. Interference and inhibition in memory retrieval. In: Bjork E.L., Bjork R.A., editors. Memory in Handbook of Perception and Cognition. Academic Press; San Diego, CA, USA: 1996. pp. 237–313.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

