Virus-Based Immuno-Oncology Models
- PMID: 35740462
- PMCID: PMC9220907
- DOI: 10.3390/biomedicines10061441
Virus-Based Immuno-Oncology Models
Abstract
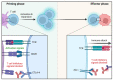
Immunotherapy has been extensively explored in recent years with encouraging results in selected types of cancer. Such success aroused interest in the expansion of such indications, requiring a deep understanding of the complex role of the immune system in carcinogenesis. The definition of hot vs. cold tumors and the role of the tumor microenvironment enlightened the once obscure understanding of low response rates of solid tumors to immune check point inhibitors. Although the major scope found in the literature focuses on the T cell modulation, the innate immune system is also a promising oncolytic tool. The unveiling of the tumor immunosuppressive pathways, lead to the development of combined targeted therapies in an attempt to increase immune infiltration capability. Oncolytic viruses have been explored in different scenarios, in combination with various chemotherapeutic drugs and, more recently, with immune check point inhibitors. Moreover, oncolytic viruses may be engineered to express tumor specific pro-inflammatory cytokines, antibodies, and antigens to enhance immunologic response or block immunosuppressive mechanisms. Development of preclinical models capable to replicate the human immunologic response is one of the major challenges faced by these studies. A thorough understanding of immunotherapy and oncolytic viruses' mechanics is paramount to develop reliable preclinical models with higher chances of successful clinical therapy application. Thus, in this article, we review current concepts in cancer immunotherapy including the inherent and synthetic mechanisms of immunologic enhancement utilizing oncolytic viruses, immune targeting, and available preclinical animal models, their advantages, and limitations.
Keywords: cancer; humanized mice; immune-oncology; immunotherapeutic; oncolytic virus; tumor microenvironment; tumor-associated macrophages; vaccinia virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

