Traumatic and Diabetic Schwann Cell Demyelination Is Triggered by a Transient Mitochondrial Calcium Release through Voltage Dependent Anion Channel 1
- PMID: 35740468
- PMCID: PMC9220872
- DOI: 10.3390/biomedicines10061447
Traumatic and Diabetic Schwann Cell Demyelination Is Triggered by a Transient Mitochondrial Calcium Release through Voltage Dependent Anion Channel 1
Abstract
A large number of peripheral neuropathies, among which are traumatic and diabetic peripheral neuropathies, result from the degeneration of the myelin sheath, a process called demyelination. Demyelination does not result from Schwann cell death but from Schwann cell dedifferentiation, which includes reprograming and several catabolic and anabolic events. Starting around 4 h after nerve injury, activation of MAPK/cJun pathways is the earliest characterized step of this dedifferentiation program. Here we show, using real-time in vivo imaging, that Schwann cell mitochondrial pH, motility and calcium content are altered as soon as one hour after nerve injury. Mitochondrial calcium release occurred through the VDAC outer membrane channel and mPTP inner membrane channel. This calcium influx in the cytoplasm induced Schwann-cell demyelination via MAPK/c-Jun activation. Blocking calcium release through VDAC silencing or VDAC inhibitor TRO19622 prevented demyelination. We found that the kinetics of mitochondrial calcium release upon nerve injury were altered in the Schwann cells of diabetic mice suggesting a permanent leak of mitochondrial calcium in the cytoplasm. TRO19622 treatment alleviated peripheral nerve defects and motor deficit in diabetic mice. Together, these data indicate that mitochondrial calcium homeostasis is instrumental in the Schwann cell demyelination program and that blocking VDAC constitutes a molecular basis for developing anti-demyelinating drugs for diabetic peripheral neuropathy.
Keywords: VDAC; mitochondria; myelin; peripheral nerve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

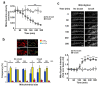
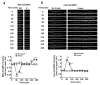
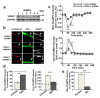


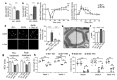
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

