Computational Detection of Extraprostatic Extension of Prostate Cancer on Multiparametric MRI Using Deep Learning
- PMID: 35740487
- PMCID: PMC9220816
- DOI: 10.3390/cancers14122821
Computational Detection of Extraprostatic Extension of Prostate Cancer on Multiparametric MRI Using Deep Learning
Abstract
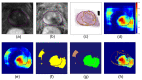
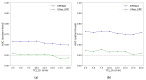
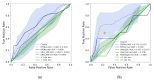
The localization of extraprostatic extension (EPE), i.e., local spread of prostate cancer beyond the prostate capsular boundary, is important for risk stratification and surgical planning. However, the sensitivity of EPE detection by radiologists on MRI is low (57% on average). In this paper, we propose a method for computational detection of EPE on multiparametric MRI using deep learning. Ground truth labels of cancers and EPE were obtained in 123 patients (38 with EPE) by registering pre-surgical MRI with whole-mount digital histopathology images from radical prostatectomy. Our approach has two stages. First, we trained deep learning models using the MRI as input to generate cancer probability maps both inside and outside the prostate. Second, we built an image post-processing pipeline that generates predictions for EPE location based on the cancer probability maps and clinical knowledge. We used five-fold cross-validation to train our approach using data from 74 patients and tested it using data from an independent set of 49 patients. We compared two deep learning models for cancer detection: (i) UNet and (ii) the Correlated Signature Network for Indolent and Aggressive prostate cancer detection (CorrSigNIA). The best end-to-end model for EPE detection, which we call EPENet, was based on the CorrSigNIA cancer detection model. EPENet was successful at detecting cancers with extraprostatic extension, achieving a mean area under the receiver operator characteristic curve of 0.72 at the patient-level. On the test set, EPENet had 80.0% sensitivity and 28.2% specificity at the patient-level compared to 50.0% sensitivity and 76.9% specificity for the radiologists. To account for spatial location of predictions during evaluation, we also computed results at the sextant-level, where the prostate was divided into sextants according to standard systematic 12-core biopsy procedure. At the sextant-level, EPENet achieved mean sensitivity 61.1% and mean specificity 58.3%. Our approach has the potential to provide the location of extraprostatic extension using MRI alone, thus serving as an independent diagnostic aid to radiologists and facilitating treatment planning.
Keywords: computer-aided diagnosis; deep learning; extraprostatic extension.
Conflict of interest statement
Mirabela Rusu is a consultant for Roche and has research grants from GE Healthcare and Philips Healthcare.
Figures






References
-
- Tourinho-Barbosa R., Srougi V., Nunes-Silva I., Baghdadi M., Rembeyo G., Eiffel S.S., Barret E., Rozet F., Galiano M., Cathelineau X., et al. Biochemical recurrence after radical prostatectomy: What does it mean? Int. Braz. J. 2018;44:14–21. doi: 10.1590/s1677-5538.ibju.2016.0656. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

