Non-Melanoma Skin Cancer Clearance after Medical Treatment Detected with Noninvasive Skin Imaging: A Systematic Review and Meta-Analysis
- PMID: 35740502
- PMCID: PMC9221328
- DOI: 10.3390/cancers14122836
Non-Melanoma Skin Cancer Clearance after Medical Treatment Detected with Noninvasive Skin Imaging: A Systematic Review and Meta-Analysis
Abstract
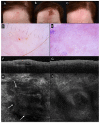
Background/objectives: Non-melanoma skin cancer (NMSC) treated with nonsurgical therapies can be monitored with noninvasive skin imaging. The precision of dermoscopy, reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) in detecting clearance is unclear. We aim to report the proportion of persisting tumors identified with noninvasive technologies available in the literature.
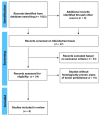
Methods: A systematic literature search was conducted on the PubMed and Cochrane Public Library Databases for articles published prior to November 2021. Statistical analyses were conducted with MedCalc 14.8.1 software.
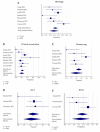
Results: A total of eight studies (352 lesions) reporting noninvasive imaging for NMSC clearance following nonsurgical treatment were included. Most (n = 7) reported basal cell carcinoma (BCC), and one study reported squamous cell carcinoma (SCC) clearance. A meta-analysis of the BCC clearance revealed that the summary effect for RCM was higher, as compared to the other techniques. Interestingly, the sensitivity and specificity for OCT were 86.4% (95% CI: 65.1-97.1) and 100% (95% CI: 94.8-100.0), respectively, whilst, for RCM, they reached 100% (95%CI: 86.8-100) and 72.5% (95% CI: 64.4-79.7), respectively.
Conclusions: Routine clinical examination and dermoscopy underperform when employed for NMSC clearance monitoring, although they represent the first approach to the patient. OCT and RCM seem to improve the detection of persistent BCC after medical treatment.
Keywords: basal cell carcinoma; dermoscopy; non-melanoma skin cancer; nonsurgical treatment; optical coherence tomography; reflectance confocal microscopy; squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

