Real-World Treatments and Clinical Outcomes in Advanced NSCLC without Actionable Mutations after Introduction of Immunotherapy in Japan
- PMID: 35740512
- PMCID: PMC9220782
- DOI: 10.3390/cancers14122846
Real-World Treatments and Clinical Outcomes in Advanced NSCLC without Actionable Mutations after Introduction of Immunotherapy in Japan
Abstract
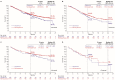
The aims of this study were to describe systemic treatment patterns and clinical outcomes for unresectable advanced/metastatic non-small-cell lung cancer (NSCLC) by first-line regimen type in real-world clinical settings in Japan after the introduction of first-line immune checkpoint inhibitor (ICI) monotherapy in 2017. Using retrospective chart review at 23 study sites, we identified patients ≥20 years old initiating first-line systemic therapy from 1 July 2017 to 20 December 2018, for unresectable stage IIIB/C or IV NSCLC; the data cutoff was 30 September 2019. Eligible patients had recorded programmed death-ligand 1 (PD-L1) tumor proportion score (TPS) and no known actionable EGFR/ALK/ROS1/BRAF genomic alteration. Kaplan-Meier method was used to determine time-to-event endpoints. Of 1208 patients, 647 patients (54%) received platinum doublet, 463 (38%) received ICI monotherapy, and 98 (8%) received nonplatinum cytotoxic regimen as first-line therapy. PD-L1 TPS was ≥50%, 1−49% and <1% for 44%, 30%, and 25% of patients, respectively. Most patients with PD-L1 TPS ≥50% received ICI monotherapy (453/529; 86%). Excluding 26 patients with ECOG performance status of 3−4 from outcome analyses, the median patient follow-up was 11.3 months. With first-line platinum doublet, ICI monotherapy, and nonplatinum cytotoxic regimens, median overall survival (OS) was 16.3 months (95% CI, 14.0−20.1 months), not reached, and 14.4 months (95% CI, 10.3−21.2 months), respectively; 24-month OS was 40%, 58%, and 31%, respectively. Differences in OS relative to historical cohort data reported in Japan are consistent with improvement over time in real-world clinical outcomes for advanced NSCLC.
Keywords: chemotherapy; immune checkpoint inhibitor; non-small-cell lung cancer; nonplatinum therapy; overall survival.
Conflict of interest statement
H.N. declares research grants to his institution from MSD K.K., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd.; and honoraria for lectures from MSD K.K., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd. T.Kijima declares research grants to his institution from Nippon Boehringer Ingelheim Co., Ltd., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Shionogi & Co., Ltd., Eli Lilly Japan K.K., MSD K.K.; and honoraria for lectures (self) from Chugai Pharmaceutical Co., LTD., Daiichi Sankyo Co., Ltd., Taiho Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., AstraZeneca K.K., Nippon Boehringer Ingelheim Co., Ltd., MSD K.K., Pfizer Japan Inc., Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K. T.Y. declares research grants to his institution from Bristol-Myers Squibb Co., Ltd., MSD K.K., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Delta-Fly Pharma, Inc.; and honoraria for lectures (self) from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co., Ltd., Ono Pharmaceutical Co., Ltd., MSD K.K., Pfizer Japan Inc., Novartis Pharma K.K., Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Eli Lilly Japan K.K. H.K. declares grants for joint research from Boeringer Ingelheim, Ono pharm., Chugai pharm.; and honoraria for lectures from AstraZeneca, Ono Pharm., Bristol Myers Squibb, Chugai pharm., Boeringer Ingelheim; and PCT issued for Biomarker to predict anticancer therapy and Immunological Biomarker to predict PD-1 ICI efficacy. T.S. has nothing to disclose. M.M. declares scholarship endowments to his institution from Chugai; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, MSD, Ono Pharmaceutical, Eli Lilly, Boehringer Ingelheim, Novartis, Chugai, Taiho, Kyowa-kirin, Otsuka, Nihon-kayaku, Pfizer, Shionogi, Daiichi-Sankyo. M.L.S. and T.B. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and stockholders of Merck & Co., Inc., Rahway, NJ, USA. K.T., T.Kamitani, M.I., and M.A. are employees of MSD K.K., Tokyo, Japan, and stockholders of Merck & Co., Inc., Rahway, NJ, USA. K.K. is an employee of MSD K.K., Tokyo, Japan. Y.G. declares grants to the clinical trial group from AZK, Pfizer; and grants to his institution from Abbvie, Eli Lilly, Pfizer, Bristol Myers Squibb, Ono, Novartis, Kyorin, DaiichiSankyo, This link Novartis, Prefered Networ; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Eli Lilly, Chugai, Taiho, Boehringer Ingelheim, Ono, Bristol Myers Squibb, Pfizer, MSD, Novartis. Merck, Thermo Fischer; and participation on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Chugai, Boehringer Ingelheim, Eli Lilly, Taiho, Pfizer, Novartis, Guardant Health Inc., Illumina, DaiichiSankyo, Ono Pharmaceutical, Bristol Myers Squibb, MSD; and leadership or fiduciary role for Cancer Net Japan and JAMT. The funders participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication.
Figures

References
-
- International Agency for Research on Cancer Cancer Today, Population Fact Sheets: Japan (Globocan 2020) [(accessed on 27 April 2022)]. Available online: https://gco.iarc.fr/today/fact-sheets-populations.
-
- Foundation for Promotion of Cancer Research Cancer Statistics in Japan. 2021. [(accessed on 27 April 2022)]. Available online: https://ganjoho.jp/public/qa_links/report/statistics/2021_en.html.
-
- Japan Center for Cancer Control and Information Services Projected Cancer Deaths in 2021. [(accessed on 27 April 2022)]. Available online: http://ganjoho.jp/en/public/statistics/short_pred.html.
-
- Sekine I., Shintani Y., Shukuya T., Takayama K., Inoue A., Okamoto I., Kiura K., Takahashi K., Dosaka-Akita H., Takiguchi Y., et al. A Japanese lung cancer registry study on demographics and treatment modalities in medically treated patients. Cancer Sci. 2020;111:1685–1691. doi: 10.1111/cas.14368. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

