Current and Emerging Methods for Ovarian Cancer Screening and Diagnostics: A Comprehensive Review
- PMID: 35740550
- PMCID: PMC9221480
- DOI: 10.3390/cancers14122885
Current and Emerging Methods for Ovarian Cancer Screening and Diagnostics: A Comprehensive Review
Abstract
With a 5-year survival rate of less than 50%, ovarian high-grade serous carcinoma (HGSC) is one of the most highly aggressive gynecological malignancies affecting women today. The high mortality rate of HGSC is largely attributable to delays in diagnosis, as most patients remain undiagnosed until the late stages of -disease. There are currently no recommended screening tests for ovarian cancer and there thus remains an urgent need for new diagnostic methods, particularly those that can detect the disease at early stages when clinical intervention remains effective. While diagnostics for ovarian cancer share many of the same technical hurdles as for other cancer types, the low prevalence of the disease in the general population, coupled with a notable lack of sensitive and specific biomarkers, have made the development of a clinically useful screening strategy particularly challenging. Here, we present a detailed review of the overall landscape of ovarian cancer diagnostics, with emphasis on emerging methods that employ novel protein, genetic, epigenetic and imaging-based biomarkers and/or advanced diagnostic technologies for the noninvasive detection of HGSC, particularly in women at high risk due to germline mutations such as BRCA1/2. Lastly, we discuss the translational potential of these approaches for achieving a clinically implementable solution for screening and diagnostics of early-stage ovarian cancer as a means of ultimately improving patient outcomes in both the general and high-risk populations.
Keywords: HGSC; biomarkers; diagnostic; emerging; ovarian cancer; screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

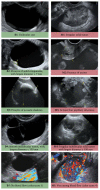




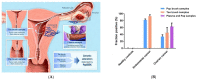
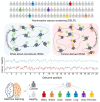

References
-
- Rendi M.H. Epithelial carcinoma of the ovary, fallopian tube, and peritoneum: Histopathology. In: Chakrabarti A., editor. UpToDate. UpToDate; Waltham, MA, USA: 2021.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

