A Platform of Patient-Derived Microtumors Identifies Individual Treatment Responses and Therapeutic Vulnerabilities in Ovarian Cancer
- PMID: 35740561
- PMCID: PMC9220902
- DOI: 10.3390/cancers14122895
A Platform of Patient-Derived Microtumors Identifies Individual Treatment Responses and Therapeutic Vulnerabilities in Ovarian Cancer
Abstract
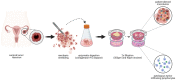
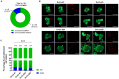
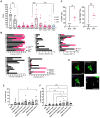
In light of the frequent development of therapeutic resistance in cancer treatment, there is a strong need for personalized model systems representing patient tumor heterogeneity, while enabling parallel drug testing and identification of appropriate treatment responses in individual patients. Using ovarian cancer as a prime example of a heterogeneous tumor disease, we developed a 3D preclinical tumor model comprised of patient-derived microtumors (PDM) and autologous tumor-infiltrating lymphocytes (TILs) to identify individual treatment vulnerabilities and validate chemo-, immuno- and targeted therapy efficacies. Enzymatic digestion of primary ovarian cancer tissue and cultivation in defined serum-free media allowed rapid and efficient recovery of PDM, while preserving histopathological features of corresponding patient tumor tissue. Reverse-phase protein array (RPPA)-analyses of >110 total and phospho-proteins enabled the identification of patient-specific sensitivities to standard, platinum-based therapy and thereby the prediction of potential treatment-responders. Co-cultures of PDM and autologous TILs for individual efficacy testing of immune checkpoint inhibitor treatment demonstrated patient-specific enhancement of cytotoxic TIL activity by this therapeutic approach. Combining protein pathway analysis and drug efficacy testing of PDM enables drug mode-of-action analyses and therapeutic sensitivity prediction within a clinically relevant time frame after surgery. Follow-up studies in larger cohorts are currently under way to further evaluate the applicability of this platform to support clinical decision making.
Keywords: RPPA protein profiling; anti-cancer drug sensitivity; cancer immunotherapy; ovarian cancer; patient-derived tumor model.
Conflict of interest statement
AH received consulting and speaking fees from GSK, AstraZeneca and Clovis. N.A., A.K., B.G., A.-L.K., A.S., S.Y.B., M.P., K.S.-L. and C.S. declare no competing interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

