Cancer-of-Unknown-Primary-Origin: A SEER-Medicare Study of Patterns of Care and Outcomes among Elderly Patients in Clinical Practice
- PMID: 35740574
- PMCID: PMC9221531
- DOI: 10.3390/cancers14122905
Cancer-of-Unknown-Primary-Origin: A SEER-Medicare Study of Patterns of Care and Outcomes among Elderly Patients in Clinical Practice
Abstract
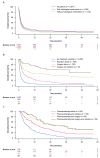
Knowledge of contemporary patterns of cancer-of-unknown-primary-origin (CUP) diagnostic work-up, treatment, and outcomes in routine healthcare is limited. Thus, we examined data from elderly patients diagnosed with CUP in real-world US clinical practice. From the Surveillance, Epidemiology, and End Results-Medicare-linked database, we included patients ≥ 66 years old with CUP diagnosed between 1 January 2013 and 31 December 2015. We analyzed baseline demographics, clinical characteristics, methods of diagnostic work-up (biopsy, immunohistochemistry, imaging), treatment-related factors, and survival. CUP diagnosis was histologically confirmed in 2813/4562 patients (61.7%). Overall, 621/4562 (13.6%) patients received anticancer pharmacotherapy; among these, 97.3% had a histologically confirmed tumor and 83.1% received all three procedures. Among those with a histologically confirmed tumor, increasing age, increasing comorbidity score, not receiving all three diagnostic measures, and having a not-further specified histologic finding of only 'malignant neoplasm' were all negatively associated with receipt of anticancer pharmacotherapy. Median overall survival was 1.2 months for all patients. Median time between CUP diagnosis and treatment initiation was 41 days. Limited diagnostic work-up was common and most patients did not receive anticancer pharmacotherapy. The poor outcomes highlight a substantial unmet need for further research into improving diagnostic work-up and treatment effectiveness in CUP.
Keywords: Medicare Part A; Medicare Part B; SEER program; diagnostic tests and procedures; drug therapy; neoplasms; registries; survival analysis; unknown primary.
Conflict of interest statement
L.M. reports travel/accommodation expenses from
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

