Phase 1/2 Trial of CLAG-M with Dose-Escalated Mitoxantrone in Combination with Fractionated-Dose Gemtuzumab Ozogamicin for Newly Diagnosed Acute Myeloid Leukemia and Other High-Grade Myeloid Neoplasms
- PMID: 35740603
- PMCID: PMC9221325
- DOI: 10.3390/cancers14122934
Phase 1/2 Trial of CLAG-M with Dose-Escalated Mitoxantrone in Combination with Fractionated-Dose Gemtuzumab Ozogamicin for Newly Diagnosed Acute Myeloid Leukemia and Other High-Grade Myeloid Neoplasms
Abstract
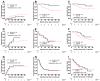
Gemtuzumab ozogamicin (GO) improves outcomes when added to intensive AML chemotherapy. A meta-analysis suggested the greatest benefit when combining fractionated doses of GO (GO3) with 7 + 3. To test whether GO3 can be safely used with high intensity chemotherapy, we conducted a phase 1/2 study of cladribine, high-dose cytarabine, G-CSF, and dose-escalated mitoxantrone (CLAG-M) in adults with newly diagnosed AML or other high-grade myeloid neoplasm (NCT03531918). Sixty-six patients with a median age of 65 (range: 19-80) years were enrolled. Cohorts of six and twelve patients were treated in phase 1 with one dose of GO or three doses of GO (GO3) at 3 mg/m2 per dose. Since a maximum-tolerated dose was not reached, the recommended phase 2 dose (RP2D) was declared to be GO3. At RP2D, 52/60 (87%) patients achieved a complete remission (CR)/CR with incomplete hematologic recovery (CRi), 45/52 (87%) without flow cytometric measurable residual disease (MRD). Eight-week mortality was 0%. Six- and twelve-month event-free survival (EFS) were 73% and 58%; among favorable-risk patients, these estimates were 100% and 95%. Compared to 186 medically matched adults treated with CLAG-M alone, CLAG-M/GO3 was associated with better survival in patients with favorable-risk disease (EFS: p = 0.007; OS: p = 0.030). These data indicate that CLAG-M/GO3 is safe and leads to superior outcomes than CLAG-M alone in favorable-risk AML/high-grade myeloid neoplasm.
Keywords: CD33; CLAG-M; acute myeloid leukemia (AML); adults; antibody-drug conjugate; chemotherapy; gemtuzumab ozogamicin; phase 1/2 trial.
Conflict of interest statement
C.D.G. received laboratory research grants and/or clinical trial support from Pfizer and ImmunoGen and has current employment and equity ownership with Bristol Myers Squibb. A.B.H. received clinical trial support from Bayer, Gilead, Imago, Incyte, Jazz, and Novartis, and is a consultant to AbbVie. J.S.A. received research support from 2seventy bio. M.-E.M.P. received clinical trial support from AbbVie, Biosight, Bristol Myers Squibb, Cardiff, Glycomimetics, Oscotec, Pfizer, and Trillium. V.G.O. received research support from Pfizer. S.B.K. is a consultant to Discmedicine. R.D.C. received clinical trial support from Amgen, Kite/Gilead, Merck, Pfizer, Servier, and Vanda Pharmaceuticals; consulting/honoraria from Amgen, Kite/Gilead, and Pfizer; serves on a data safety monitoring board for Pepromene Bio; and his spouse is employed by and owns stock in Seagen. R.B.W. received laboratory research grants and/or clinical trial support from Agios, Amgen, Aptevo, Arog, BioLineRx, Celgene, ImmunoGen, Janssen, Jazz, Kura, MacroGenics, Pfizer, Selvita, and Stemline; has ownership interests in Amphivena; and is (or has been) a consultant to Agios, Amgen, Amphivena, Aptevo, Astellas, BioLineRx, Boston Biomedical, Bristol Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Janssen, Jazz, Kite, Kronos, MacroGenics, New Link Genetics, Pfizer, and Race. The other authors declare no competing financial interests.
Figures


References
-
- Burnett A.K., Hills R.K., Milligan D., Kjeldsen L., Kell J., Russell N.H., Yin J.A., Hunter A., Goldstone A.H., Wheatley K. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: Results of the MRC AML15 trial. J. Clin. Oncol. 2011;29:369–377. doi: 10.1200/JCO.2010.31.4310. - DOI - PubMed
-
- Delaunay J., Recher C., Pigneux A., Witz F., Vey N., Blanchet O., Lefebvre P., Luquet I., Guillerme I., Volteau C., et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation. Results of the GOELAMS AML 2006 IR study [abstract] Blood. 2011;118:37–38. doi: 10.1182/blood.V118.21.79.79. - DOI - PubMed
-
- Castaigne S., Pautas C., Terré C., Raffoux E., Bordessoule D., Bastie J.N., Legrand O., Thomas X., Turlure P., Reman O., et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. - DOI - PubMed
-
- Burnett A.K., Hills R.K., Hunter A.E., Milligan D., Kell W.J., Wheatley K., Yin J., McMullin M.F., Dignum H., Bowen D., et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: Results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013;27:75–81. doi: 10.1038/leu.2012.229. - DOI - PubMed
-
- Petersdorf S.H., Kopecky K.J., Slovak M., Willman C., Nevill T., Brandwein J., Larson R.A., Erba H.P., Stiff P.J., Stuart R.K., et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

