A Tale of Two Cancers: A Current Concise Overview of Breast and Prostate Cancer
- PMID: 35740617
- PMCID: PMC9220807
- DOI: 10.3390/cancers14122954
A Tale of Two Cancers: A Current Concise Overview of Breast and Prostate Cancer
Abstract
Cancer is a global issue, and it is expected to have a major impact on our continuing global health crisis. As populations age, we see an increased incidence in cancer rates, but considerable variation is observed in survival rates across different geographical regions and cancer types. Both breast and prostate cancer are leading causes of morbidity and mortality worldwide. Although cancer statistics indicate improvements in some areas of breast and prostate cancer prevention, diagnosis, and treatment, such statistics clearly convey the need for improvements in our understanding of the disease, risk factors, and interventions to improve life span and quality of life for all patients, and hopefully to effect a cure for people living in developed and developing countries. This concise review compiles the current information on statistics, pathophysiology, risk factors, and treatments associated with breast and prostate cancer.
Keywords: breast cancer; cancer classification; cancer diagnosis; cancer heterogeneity; cancer pathophysiology; cancer statistics; cancer treatment; drug-tolerant persister cells; female breast anatomy; multidrug resistance; neuroendocrine cancer; prostate anatomy; prostate cancer; risk factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

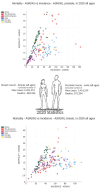
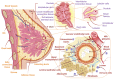



References
-
- Hassanpour S.H., Dehghani M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017;4:127–129. doi: 10.1016/j.jcrpr.2017.07.001. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

