Unraveling the Biology of Epithelioid Hemangioendothelioma, a TAZ-CAMTA1 Fusion Driven Sarcoma
- PMID: 35740643
- PMCID: PMC9221450
- DOI: 10.3390/cancers14122980
Unraveling the Biology of Epithelioid Hemangioendothelioma, a TAZ-CAMTA1 Fusion Driven Sarcoma
Abstract
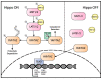
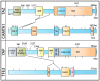
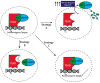
The activities of YAP and TAZ, the end effectors of the Hippo pathway, are consistently altered in cancer, and this dysregulation drives aggressive tumor phenotypes. While the actions of these two proteins aid in tumorigenesis in the majority of cancers, the dysregulation of these proteins is rarely sufficient for initial tumor development. Herein, we present a unique TAZ-driven cancer, epithelioid hemangioendothelioma (EHE), which harbors a WWTR1(TAZ)-CAMTA1 gene fusion in at least 90% of cases. Recent investigations have elucidated the mechanisms by which YAP/TAP-fusion oncoproteins function and drive tumorigenesis. This review presents a critical evaluation of this recent work, with a particular focus on how the oncoproteins alter the normal activity of TAZ and YAP, and, concurrently, we generate a framework for how we can target the gene fusions in patients. Since EHE represents a paradigm of YAP/TAZ dysregulation in cancer, targeted therapies for EHE may also be effective against other YAP/TAZ-dependent cancers.
Keywords: TAZ–CAMTA1; YAP/TAZ; epithelioid hemangioendothelioma; hippo pathway; sarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

